İçindekiler
- 1 DERMAL FILLER COMPLICATIONS
- 1.1 ABSTRACT
- 1.2 A doctor should know how to identify early and late onset complications.
- 1.3 METHODOLOGY
- 1.3.1 1.1 Etiology and Classification of Patient Dissatisfaction
- 1.3.2 1.2 Classification of Complications
- 1.3.3 1.3 Bruising and Swelling
- 1.3.4 1.3.1 Treatment
- 1.3.5 1.3.2 Prevention
- 1.3.6 1.4 Erythema
- 1.3.7 1.4.1 Etiology
- 1.3.8 1.4.2 Treatment
- 1.3.9 1.5 Infection
- 1.3.10 1.5.1 Symptoms
- 1.3.11 1.5.1 Treatment
- 1.4 It is very important that the wound does not dry out.
- 1.5 Nevertheless, we must inject hyaluronidase.
- 1.5.1 1.8 Migration
- 1.5.2 1.9 Transparent Effect and Tyndall Effect
- 1.5.3 1.9.1 Cause
- 1.5.4 1.9.2 Location
- 1.5.5 1.9.3 Prevention and Treatment
- 1.5.6 1.10 Skin Marking
- 1.5.7 1.11 Filler-Induced Hypersensitivity Inflammation and Granuloma
- 1.5.8 2.1 Hyaluronic Acid
- 1.5.9 2.2 HA Filler
- 1.5.10 2.3 HA Filler Manufacturing Process
- 1.5.11 2.4 Properties of HA Fillers
- 1.5.12 2.4.1 Biphasic Versus Monophasic
- 1.5.13 2.4.2 HA Concentration
- 1.5.14 2.4.3 Particle Size
- 1.5.15 2.4.4 Injection Force, Extrusion Force
- 1.5.16 2.4.5 Cross-Linking Ratio, Degree of Modification (MOD)
- 1.5.17 2.4.6 Rheology
- 1.5.18 2.4.7 Cohesiveness
- 1.5.19 2.5 Hyaluronidase
- 1.5.20 3.1 Filler-Induced Hypersensitivity Inflammation
- 1.5.21 3.1.1 Pathophysiology
- 1.5.22 3.1.2 Symptoms
- 1.5.23 3.1.3 Differential Diagnosis
- 1.5.24 3.1.4 Treatment
- 1.5.25 3.2 Filler Granuloma
- 1.5.26 3.2.1 Pathophysiology
- 1.5.27 3.2.2 Classifications
- 1.5.28 3.2.3 Treatments
- 1.5.29 4.1 Facial Danger Zones
- 1.5.30 4.1.1 Thick Skin Area
- 1.5.31 4.1.2 Subcutaneous Layer
- 1.5.32 4.1.2.1 The Supraorbital Artery
- 1.5.33 4.1.2.2 The Supratrochlear Artery
- 1.5.34 4.1.2.3 The Lateral Nasal Artery
- 1.5.35 4.1.2.4 The Dorsal Nasal Artery
- 1.5.36 4.1.2 Isolated Area
- 1.5.37 4.1.3 Foramen
- 1.5.38 4.2 Safe Zones
- 1.5.39 4.3.1 Glabella
- 1.5.40 4.3.2 The Forehead
- 1.5.41 4.3.3 The Nasal Root
- 1.5.42 4.3.4 Nasal Tip
- 1.5.43 4.3.5 Ala Nasi (Wing of the Nose)
- 1.5.44 4.3.6 Infraorbital Foramen
- 1.5.45 4.3.7 Nasolabial Fold
- 1.5.46 4.3.8 Temple
- 1.5.47 5.1 Skin Necrosis Definition and Mechanism
- 1.5.48 5.1.1 Skin Necrosis Definition
- 1.5.49 5.1.2 Mechanism
- 1.5.50 5.2 Classification of Skin Necrosis
- 1.5.51 5.3 Localized Skin Necrosis
- 1.5.52 5.3 Localized Skin Necrosis
- 1.5.53 5.3.1.1 Decompression
- 1.5.54 5.3.1.2 Pustule Removal
- 1.5.55 5.3.1.3 Closed Wet Dressing
- 1.5.56 5.3.1.4 Treatment After Acute Stage
- 1.5.57 5.3.1.5 Misunderstanding Treatment
- 1.5.58 5.4 Extended Necrosis
- 1.5.59 5.4.1 Proximal Necrosis
- 1.5.60 5.4.2 Distant Necrosis
- 1.5.61 6.1 Incidence of Ocular Complications
- 1.5.62 6.2 Pathophysiology
- 1.5.63 6.3 Symptoms
- 1.5.64 6.3 Treatments
- 1.5.65 6.3.1 Emergency Treatment
- 1.5.66 6.3.2 Retrobulbar Hyaluronidase Injection
- 1.5.67 6.3.3 Emergency Kit
- 1.5.68 6.5 Prevention
- 1.5.69 6.5.1 Anatomy
- 1.5.70 6.5.2 Aspiration
- 1.5.71 6.5.3 Big Cannula/Needle
- 1.5.72 6.5.4 Compression
- 1.5.73 6.5.5 Direction
- 1.5.74 6.5.6 Epinephrine
- 1.5.75 6.5.7 Filler Injection Technique
- 1.5.76 6.5.8 Gentle Injection
- 1.5.77 6.5.9 History
- 1.5.78 6.5.10 Injection by Cannula or Needle
- 1.6 RESULT
- 1.7 CONCLUSION
- 1.8 REFERENCES
DERMAL FILLER COMPLICATIONS
– CAMERINO UNIVERSITY MEDICAL FACULTY
– ROMA, ITALY
– Dr. Şafak Göktaş
December 2022
ABSTRACT
Dermal filler applications have become very popular in recent years. With the increase in people’s economic purchasing power and giving more importance to beauty, more people turned to filler applications. It is possible to get results in a short time with the operations known as a ‘small touch’. Dermal fillers are at the forefront of these procedures. Such procedures are very simple for both the doctor and the patient. But it is very important to have anatomical knowledge to know the characteristics of the filler and the application technique.
As a result of the correct application of the filler injecting process, the quality of life and visuality of the patient can improve in a good way. But as a result of careless applications, some complications may develop and cause disappointment for the patient. Therefore, in the face of a potential complication, the doctor should be able to make a good complication management. Minor complications such as swelling, redness and bruising may be seen. However, other than that, serious complications may result, even blindness may develop.
A doctor should know how to identify early and late onset complications.
The majority of complications are related to accepting inappropriate patients for treatment, issues of sterility, placement, volume and injection tecnique. Complications can be avoided with excellent procedure tecnique, knowledge of facial anatomy, proper patient selection and appropriate pre and post skin care.
All aesthetic practitioners will face complications during their career. It is therefore vital to be prepared. A complication is so named because it complicates the situation. The more invasive the treatment, the greater the severity of the risk. Danger zones needed to be known. Careful assessment, history taking, diagnosis, planning and execution of the treatment are all important factors to reduce the risk of complications. A doctor should know how to identify early and late onset complications.
We must be open and honest with our patients, our colleagues and ourselves when things go wrong. Because things will go wrong. We must know how to recognise them when they do, correct them or seek help if we can’t.
I believe that all medical aesthetic practioners must know the possible complications and know how to manage them. That was my purpose to chose this topic. In this thesis, I tried to summarise the complicaitons of medical aesthetics. My aim is to get attention to the complicaitons and its management.
METHODOLOGY
1.1 Etiology and Classification of Patient Dissatisfaction
Filler injections are one of the indispensable procedures of the medical aesthetic field. The usage has increased visibly in recent years. In some cases, filler injections have become preferred instead of surgical procedures. For example, filler injections into the nose instead of rhinoplasty are very popular lately. However, filler complications increase due to the increased number of filler injections. Therefore, practitioners should be aware of complications such as skin necrosis and blindness.
We can classify the causes of patient dissatisfaction as follows:
1. Medical Malpractice,
2. Patient not following the instructions
3. Characteristics of the filler
4. Patient’s subjective point of view,
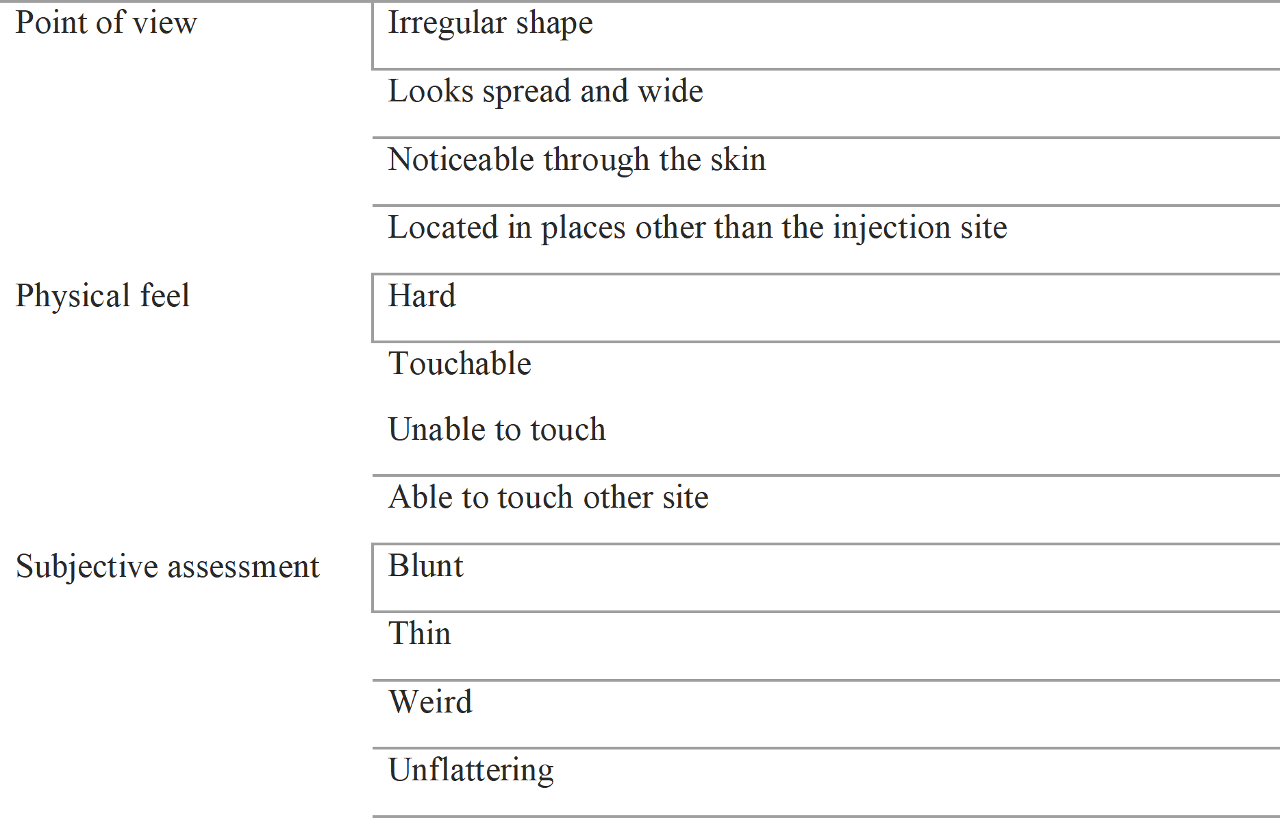
Point below, you can see the table that classifies patient dissatisfaction
Dissatisfaction in the patient may be due to more than one reason. For example, an irregular and bumpy appearance may be due to medical malpractice. But it may also develop as a result of problems related to the character of the filler. So, patients must be photographed before the procedure.
1.2 Classification of Complications
The most important issue for complication is the onset time. The point can show us some clues for the right treatment. ( Table 1.2 )
Table 1.2 Complication classified by onset time
1.3 Bruising and Swelling
Bruising is the most common simple complication. The color of the bruise can vary from red to yellow and even green. Vascular damage and blood pooling cause bruising. Bruising sometimes can appear below the injection point because of the gravity.
If there is severe bleeding, then bruising and stiffness may be felt together. The bruising in calcium hydroxyapatite or polycaprolactone fillers may be larger and longer lasting. If the bruising exceeds 48 hours then medical support may be required.
1.3.1 Treatment
There are many methods to reduce bruising. Creams containing vitamin K or phototherapy may be recommended. Ice application is recommended in the clinic, immediately after the procedure. But it is not recommended to continue ice application at home because the filler might be excessively compressed.
1.3.2 Prevention
There are many techniques to prevent bruising. One of them is to give the filler from one point in a linear retrograde manner. The more points we inject, the greater the possibility of bruising. After the needle tip enters the skin, the movement should be minimal so that the vascular and tissue damage is minimal. It is important that the needle tip is in the vein free part under the skin. For example, when filling the nose, it will be more meaningful to give it to the supraperiosteal layer which is relatively more reliable because there is less vascularity in this area.
1.4 Erythema
It is normal to have temporary erythema within 10 minutes after the procedure. If it lasts longer than 24 hours, then it should be taken seriously. It should be considered that there may be a circulatory disorder in that area due to the filler. The pressure of the filler on the veins can reduce the blood flow to that area. We consider erythema as a minor complication, but increased compression pressure may cause skin necrosis. Therefore, it is necessary to closely observe the erytema.
1.4.1 Etiology
Erytema appears more frequently in areas with excess skin. For example, after the filler injection applied to the dorsum of the nose, the pressure spreads to the surrounding tissues. On the contrary, since a single point is affected in the filler applied to the tip of the nose, this pressure causes eryteme at the injection point. If there is a previous scar on the skin, there can be vascular microcirculation problem there. If there is a implant, a capsule may form around an implant that can cause serious problem.
Some semi permanent fillers, polymethylmethacrylate (PMMA) or calcium hydroxyapatite fillers may form a separate layer and disrupt circulation. Care should be taken when applying such fillers.
1.4.2 Treatment
The main purpose of the treatment is to reduce the pressure. Since it is caused by a complete circulatory disorder, reducing the compression pressure is the most important step in preventing skin necrosis. In these cases, aggressive composition reducing treatment should be applied.
1. Sudden whitening
2. Progressive erythema that continues 10 minutes after the procedure
3. Severe sensitivity at injection points
4. Progressive erythem and persistence of pain 2 days after the procedure
There are various methods to reduce the pressure.
The most important of which is to give the Hyalurinidase enzyme that breaks the Hyaluronic acid down to the parts. When the decision to dissolve the filler is made, it is important to use enough amount of Hyalurinidase. It may be dangerous not to go to a part of the filler and skin necrosis may develop. Therefore, it is recommended to dissolve the entire filler.
In semi permanent fillers such as PMMA and polyacrylamide, aspiration using an 18g needle tip is recommended. In calcium hydroxyapatite fillers, liquid formation is preserved for 2 weeks after the procedure. As time passes, calcium hydroxyapatite fillers become solid. Therefore, fillers should be removed within two weeks. If it exceeds 2 weeks, the filler will gradually harden and surgery will be required to remove. Over time, the filler forms hard particles like an artificial bone, integrates with normal tissue and become quite difficult to remove.
The use of antibiotics and anti inflammatory drugs is important to reduce severe ischemic damage. Small ischemic damage may be accompanied by erythema. However, if ischemic damage is severe, it may be accompanied by epithelialization disorder and skin infection.
1.5 Infection
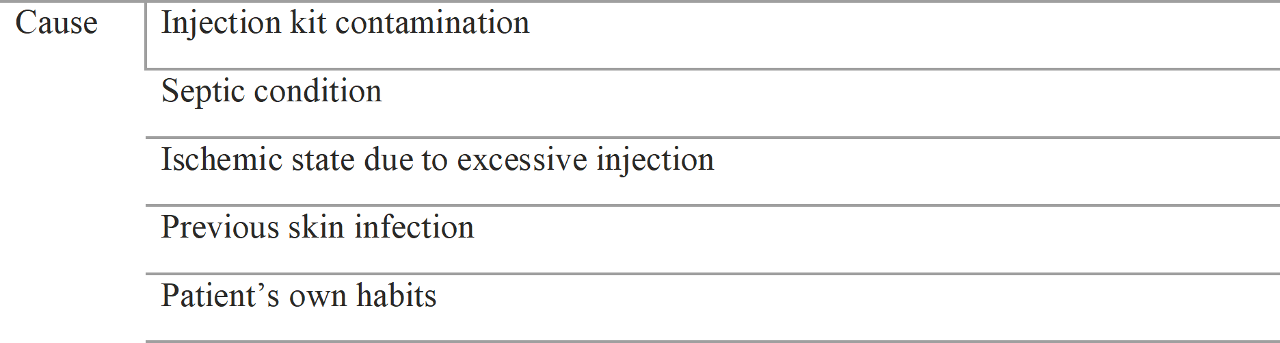
There are many causes of infection. ( Table 1.3 )
Table 1.3 Etiology of infection
Contamination of the syringe or needle tip is extremely rare. If the injection kit is contaminated, the filler is also contaminated and soft tissue infection is inevitable. Infection may occur in muliple areas, especially with the needle entering and exiting the skin multiple times.
In order to prevent this, the number of injections should be reduced. If necessary, the needle tip should be changed during the injection. Contamination may occur during the filler preparation process, for this aseptic preparation conditions should be established. Filler injection should be avoided in areas with previous skin infection or inflammation. After the procedure, patients should be warned not to compress, massage or touch the filled area. The most common way of talking about infections is after ischemic vascular events. Therefore, if erythema lasts for more than two days and there are signs of infection, treatment should be started quickly.
1.5.1 Symptoms
Infections can be categorized as general infections or due to vascular compression. Infections due to ischemic change are usually associated with overfilling. Erythema can grow as a natural consequence of post injection circulatory deterioration.
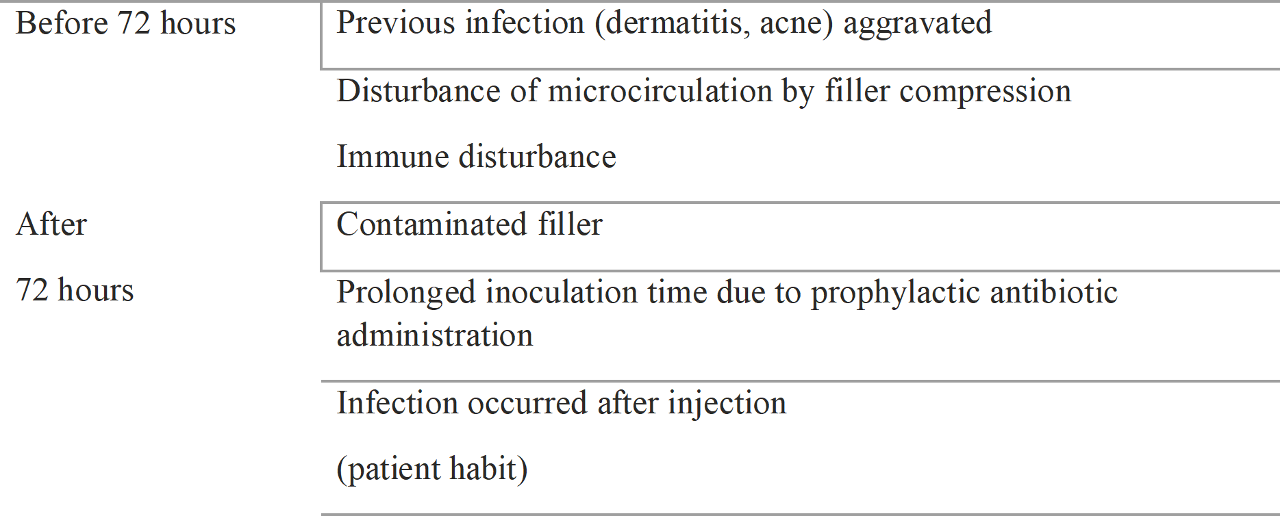
If the erythema persists for a long time and the infection occurs after 48 hours, cause of the infection is microcirculation disorder. If the infection does not occur after erythema, it is a general infection. In both cases signs of infection appear after 48 hours. Time variations occur according to compression severity. In cases of severe vascular compromise, infection sign might occur in 36 hours. If it is not severe, infection signs might occur minimally after 72 hours. General infections tend to occur 2–5 days after injection because of the incubation period. However, if infection occurs before 72 hours, it is likely because of ischemic problem; if it occurs after 72 hours, it is likely due to a general infection.
Infection onset time is very important for distinguishing the cause of an infection. Causes of infection onset occurring before or after 72 hours are detailed as follows (Table 1.4)
Table 1.4 Cause of infection
1.5.1 Treatment
Minor infections would be cured by preventive antibiotics, but if the filler is contaminated, it must be removed. Once the filler is determined to be contaminated, it should be treated as an infected foreign body. Antibiotics cannot reach the pathogen because the filler acts as a barrier and prolongs the infection. Thus, if there is any suspicion of infection, potent antibiotics like quinolone are needed; if there are signs of a prolonged infection, the filler must be removed. We recommend filler removal and immediate antibiotics administration if any signs of infection are seen.
The most important step is removing the cause of the infection when it occurs due to ischemic change. Thus, the most important treatment is decompression. One concern is that the iatrogenic spread of infection after hyaluronidase injection might destroy the inflammatory wall. Thus, when we inject hyaluronidase, inject exact layer of filler exist and also dilute half dose of normal saline to minimized connection of infection. Another important thing is minimizing the number of injections and tissue damage.
Pustules appear 48 hours after injection and spread and worsen as infection occurs. Pustules appear due to disruption of the skin’s defense mechanism due to ischemic damage and normal modifications of the flora to toxic pathogens. Pustule treatment should include careful drainage. “Careful” means removing the pustule with minimal damage to adjacent tissue. This adjacent tissue should not be destroyed as it will return to normal after the pustule is removed. These tissues are fragile due to microcirculation disorder. Vigorous manipulation causes skin peeling, scarring and tissue loss. Therefore, careful manipulation with light pressure is necessary to remove the pus contained in the subcutaneous layer.
It is very important that the wound does not dry out.
After 48 hours, the pustule may intensify, so a dressing change and evacuation of the pustule should be made twice a day to prevent tissue damage. First, we drain the pustule first and apply antiseptic and Vaseline ointment gauze to prevent wound drying. Vaseline ointment prevents the gauze from sticking to the wound to prevent skin damage during removal. Applying an antiseptic such as povidone iodine can be toxic to the wound, so consider its application in cases of severe infection.
It is very important that the wound does not dry out. Wounds tend to dry out when a pustule is not properly removed or a dressing is not applied. As the wound dries, the discharge pus turns into a harder crust of tissue. This crusty tissue impairs pus drainage and wound healing. It is therefore very important that the wound does not dry out and when a crust appears it should be removed very carefully using antiseptics such as hydrogen peroxide.
If the dressing and infection control are done correctly, it is possible for the wounds to heal within 7 days. After 7 days, hyperpigmentation begins to appear due to tissue damage. Hyperpigmentation may worsen within 2 months, but after 3-4 months of ultraviolet (UV) protection, pigmentation is likely to return to normal. For this reason, it is recommended to apply UV protection cream at the first stage and avoid laser treatment.
Recently, some new treatments such as stem cell transplantation, platelet-rich protein (PRP) and epidermal growth factor (EGF) have been introduced, but such treatments are not recommended at the infection stage. These treatments can accelerate wound healing.
1.6 Skin Necrosis
Skin necrosis is one of the tragic complications of filler injection.
1.6.1 Cause
Injected fillers disturb the circulation and skin necrosis results from ischemic damage. Ischemic damage causes infection and progression to infectious necrosis. The most mild phenomenon is erythema, while the most severe is skin necrosis.
1.6.2 Symptoms
Erythema is the earliest symptom of skin necrosis. Blanching may be easily missed and easy to ignore because it tends to redden immediately. Local anesthetic creams or injections tend to make skin whiter than the surrounding areas. The important thing to note is that, after being reddish, instead of normalizing, the skin tends to develop a red wine color. This is the first symptom of disturbed circulation. This symptom fades gradually within 48 hours or rapidly progresses within 6 hours.
Reduced circulation causes ischemic damage and the tendency to progress to liquefaction and permanent damage. At this moment, the normal defense mechanisms might fail, and normal flora of the skin attacks then progresses to infectious necrosis. This usually starts within 48 hours, but severe compression could become evident within 36 hours.
Infectious necrosis begins with pus at the hair follicle; if it is unable to drain, the infection spreads to the subcutaneous tissue and aggravates the necrosis. A depressed scar may then form because of subcutaneous tissue destruction.
The red wine color indicates severe damage, while vasodilation appears red and orange. The wound is likely to be a scab because of dryness if a proper dressing cannot prevent wound drying. Pus under a scab tends to indicate more severe damage.
After infectious necrosis, permanent skin damage can occur. Scar tissues form at the skin damage site and affect the adjacent tissues by a process called scar contracture.
1.6.3 Treatment
Determining the stage of necrosis and responding quickly lead to a better prognosis. Decompression is the principle of necrosis treatment, and proper decompression determines the prognosis. When proper decompression is performed, then proper infection control is possible.
Severe necrosis does not occur when proper treatment is provided at the stage of ischemic necrosis or infectious necrosis. However, if necrosis occurred because of delayed treatment, there is a need to consider adjuvant therapy such as stem cell treatment, PRP, EGF, and polydeoxyribonucleic acid for wound healing.
Wide debridement or skin grafting could expedite wound healing, but it is not recommended because of the risk of disastrous aesthetic consequences.
1.7 Vascular Obstruction
Vascular obstruction results in localized or wide spread phenomena. Severe complications such as blindness and cerebral embolism might happen due to extensive vascular obstructions.
1.7.1 Etiology
Localized vascular obstruction is usually caused by compression rather than filler embolism. In such cases, it is usually not affected by main vessels; rather, it is affected by small vessels and the vascular network, which is more superficial than the subcutaneous tissue and tends to be compressed.
When the main vessels are obstructed, symptoms tend to be more extensive and affected where the vessels are arborized. Main vessel obstructions are caused by emboli or compression. Main vessel obstructions also tend to be compressed, but they are usually located in the deeper subcutaneous layer, and vessel blood pressure is higher and less subjective to compressive obstruction.
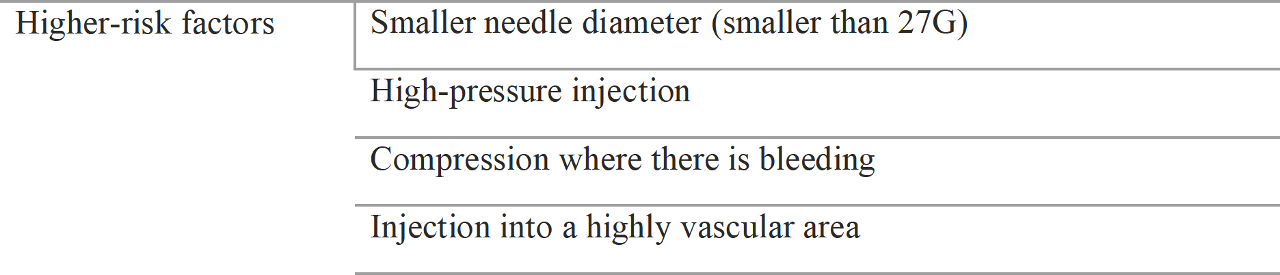
The most serious problem is when fillers are injected directly to vessels and emboli run to the ocular vessels or brain vessels. If filler is injected with enough pressure to regurgitate, filler emboli run to ocular arteries or brain arteries. Table 1.6 demonstrates the risk factors of filler emboli.
The possibility of filler injection into a vessel is higher when the needle diameter is smaller. This is the same mechanism as with intravenous injection procedure, in which it is easier to inject into a vein with smaller needle. Thus, the injection of filler with a small diameter needle could create an embolism because of the ease of injecting into a vessel and the relative higher pressure to extrude the filler.
Table 1.6 Risk factors of filler embolism
1.7.2 Symptoms
1.7.2.1 Localized Vascular Occlusion
Localized vascular occlusion usually occurs because of subcutaneous vascular network compression by the filler injection. Symptoms are localized, and the most severely compressed region tends to be the most reddish. The extent of redness depends on compression severity. Such areas tend to become blanched immediately. Localized vascular network compression results in blanching ischemic change, which promotes vasodilating mediators such as histamine release and color changes to red and a red wine color. If the pressure does not subside, infectious necrosis develops after 48 hours.
1.7.2.2 Extensive Vascular Occlusion
Extensive vascular occlusion occurs when the main vessels are obstructed by compression or embolism. It affects deeper vessels than in localized occlusion cases. It appears as the vessels are arborized. This is because relatively larger vessels are first affected, followed by other branched vessels. This is likely to feature inflammation at the injection site but might affect ischemic damage at distant lesions. It is likely to feature more extensive blanching lesions compared to localized occlusions and have a reticular pattern because of the vascular territory. If it continues, compression is likely to progress to the infectious stage.
1.7.2.3 Distant Vessel Obstruction
Vessel obstruction at a distant location occurs when filler is injected into anatomically well known arteries. The locations of the injection site and affected arteries are described in Table 1.7
Table 1.7 Arteries associated with filler embolism
Filler injected into these arteries could overcome arterial pressure and regurgitate to the ophthalmic arteries or cerebral arteries and cause blindness or brain infarction. Complication symptoms can be seen immediately such as blindness or neurological signs and should be treated immediately because these are the most emergent situations. However, in reality, there are no specific treatments for these situations.
1.7.3 Treatment
1.7.3.1 Localized Vessel Obstruction Treatment
Prognosis depends on how fast decompensation can occur. When injecting hyaluronic acid filler, hyaluronidase should be injected to provide decompensation. Hyaluronidase contains 1500 IU in each vial and is usually mixed with 1–1.5 cc of normal saline. When ischemic complications occur, the injection of one vial of hyaluronidase is recommended. For example, when compression of the nasal tip is suspected after the injection of 0.1–0.2 cc of filler, 0.5 cc of hyaluronidase should be administered and massaged very gently. Massage is needed to spread the hyaluronidase because the filler will not degrade otherwise. However, it should be done very gently to prevent the destruction of destroy fragile tissues.
A problem occurs in cases of delayed detection of ischemic changes and the progression to necrosis. Nevertheless, we must inject hyaluronidase. However, we should carefully inject hyaluronidase in cases of wound infection because the infection might spread. Thus, it is recommended that a half dose of normal saline be mixed with hyaluronidase and be injected at the exact filler location. The pustule should be removed before the hyaluronidase injection and care taken to prevent damage to the normal fragile tissue.
Nevertheless, we must inject hyaluronidase.
Even damaged tissues should be preserved whenever possible rather than debrided or removed. These fragile tissues act as a framework of the wound healing process, and the viability of this tissue is important for prognosis. Thus, it is important to preserve viable tissue whenever possible, remove the pustules very carefully, and cover the wound with Vaseline gauze. As mentioned before, if the wound is dried, a scab is created, and pus cannot drain, so a depressed scar would occur; thus, a wound should not be allowed to dry.
Antiseptics such as povidone-iodine are quite toxic and should be used minimally in cases of definite infectious signs.
When applying a dressing, clean all exudates and drain the pustules, and then cover the wound with Vaseline gauze to prevent the dried gauze and exudate from sticking together. Changing of the Vaseline gauze should be done until there are no pustule and exudate. If the infection has subsided, the dressing should be minimal to promote wound healing. Dressings should cover the wound widely to protect the fragile tissues.
Dressing should be done until complete reepithelization occurs. Patients should be educated about how to prevent hyperpigmentation. Post inflammatory hyperpigmentation might occur until 2 months, but it usually recovers to normal. However, UV exposure can prolong hyperpigmentation, so it is important to apply UV protection cream.
1.7.3.2 Extensive Vascular Obstruction Treatment
When suspected, immediate decompression and aseptic dressing can be used to achieve a full recovery. Aggressive treatment might lead to better results than localized compression because it uses collateral circulation.
1.7.3.3 Distant Vascular Obstruction Treatment
Visual disturbance or cerebral infarction patients should be transferred immediately. Retrobulbar hyaluronidase injections were recently proposed for treatment, but they are not yet definite. Ocular massage has also been proposed, but scientific evidence of this is lacking.
1.8 Migration
Filler usually remains where it is injected, but it can migrate. This phenomenon can be divided into immediate migration and delayed migration.
1.8.1 Cause
1.8.1.1 Immediate Migration
Immediate migration is usually a result of medical malpractice, i.e., a high pressure injection without external guide. That means that we can prevent filler migration by making a guide with external pressing.
When filler is injected, it is likely to move to the tissue with the lowest resistance. This hap- pens when the injection is highly pressurized. For example, when filler is injected to correct nasolabial folds, it tends to spread above the nasolabial fold because those tissues are softer. If the injected pressure is higher, filler might enter the posterior part of the maxillary bone. Thus, nasolabial fold correction should be performed while pressing on the areas to which spreading is not desired. The clinician must check every minute whether the filler is properly lifting the soft tissues.
Amazingly, filler is sometimes found in distant places. One patient who underwent nasolabial fold correction displayed swelling of the upper lips after injection. Apparently a tunnel was created through the subcutaneous layer through which the injected filler migrated. The filler was immediately removed from the upper lip, but the rest of the filler had to be removed as well since it was likely to migrate through the tunnel over time.
Thus, it is very important to check between the procedures and provide guidance with external handling. Injections made with smaller needles require more pressure, so they should be performed more carefully.
Tissue density is also important. Soft tissues tend to have high lifting capacity with low injection pressure, which minimizes the possibility of migration. However, very dense tissues require high pressure and a larger volume to create lift, which increases the risk of migration.
1.8.1.2 Delayed Migration
Patient Manipulation
The most common reason of filler migration is patient manipulation. Filler is basically viscous material that can change shape when compressed. Patient may attempt to mold the area as the doctor does immediately after the injection, which may induce migration.
A depressive finding follows filler migration because of loss of the initial lifted volume. This might occur with highly cohesive fillers because filler should be well integrated with the tissue rather than cohesive with itself.
The nose, nasolabial fold, forehead, and chin are the places that patients are likely to compress. Thus, after filler injection, it is quite important to warn patients that it might migrate when compressed.
Migration due to Filler Properties
Migration due to properties of different fillers usually occurs at the nose or chin, where it is expected to maintain shape against high pressure. When soft fillers are used in these areas, the initial shape is good, but the look spreads and widens over time. Injected filler in the chin area tends to migrate with mentalis muscle action.
This phenomenon can also be seen at the nose with the use of soft fillers. The nose is usually divided into the nasal root, supratip depression, and nasal tip.
Over time, filler injected into the supra tip depression tended to migrate to the nasal root or nasal tip. This phenomenon occurred because this area was thinner than the soft tissue at the nasal root or tip. This phenomenon occurs gradually and can be seen by permanent fillers compared to hyaluronic acid fillers.
Horizontal and perpendicular migration can occur. This is usually seen at the nasal tip, i.e., the first injected area, which was superficial to the alar cartilage but migrated to the soft tissue. This might be seen when the skin is relatively not dense or a patient squeezes the nasal tip.
Migration due to Muscle Action
Migration due to muscle action is typically seen when filler is injected into the forehead and frontalis muscle action and corrugator supercilii muscle action induces migration.
Photograph should be taken of the patients making facial expressions. Irregularities in this area should be compared to this photograph, and botulinum toxin should be injected to correct the issue.
1.9 Transparent Effect and Tyndall Effect
The transparent effect is that injected filler can be seen through thin skin. If the filler has color itself, it is likely to be visible. Colored fillers include calcium hydroxyapatite filler (white), collagen filler (yellow), and polycaprolactone filler (white)
Most fillers are colorless. In addition to a transparent effect, a Tyndall effect is visible.
★ Tyndall effect: Light scattering by particles in a colloid or very fine suspension. Fillers under the skin tend to scatter light and appear blue.
1.9.1 Cause
The Tyndall effect is seen when inject transparent filler is injected into the superficial layer of thin skin. The more filler is injected, the higher the risk of the Tyndall effect due to greater reflection of the medium. The only prevention consists of not injecting filler superficially and injecting only small amounts of filler. Thus, clinicians must be careful about skin thickness and regulate the amount of filler used.
1.9.2 Location
The Tyndall effect can occur when filler is injected into thin skin, particularly in the pretarsal area, tear trough, lips, and nose. The nose contains relatively thick skin, but when large volumes of filler are used, greater reflection of medium can occur.
The pretarsal region is where the Tyndall effect frequently occurs. For prevention, it is better to inject fillers into deeper layers than the orbicularis oculi muscle. Since the pretarsal portion of the orbicularis oculi muscle is very thin, one should inject the filler onto the tarsal plate. However, the filler could migrate to the subcutaneous layer, so it is important to warn patients about the Tyndall effect.
To avoid the Tyndall effect at the tear trough, do not inject filler into the superficial layer; rather, inject it to correct the deep groove. Educate the patient about the Tyndall effect prior to make the injection.
The Tyndall effect can occur at the nose after a superficial injection. Upon the injection of large volumes of filler, the Tyndall effect can occur. The dorsum of the nose is particularly susceptible because the skin there is relatively thinner and a large volume is injected. When injecting a large volume at the superficial nasal tip, the Tyndall effect might be seen. However, filler might migrate superficially when it is injected into the deep layer above the interdomal alar cartilage.
The Tyndall effect might also occur at the lips because of thin skin and mucosa. When filler is injected into the submucous layer, transparency is higher at the mucosa, making the Tyndall effect more common.
1.9.3 Prevention and Treatment
Filler removal is key to treatment. Hyaluronic acid filler is removed by hyaluronidase, while colored fillers such as calcium hydroxyapatite filler, polycaprolactone filler, and the collagen filler and permanent fillers such as polyacrylamide gel filler should be removed by aspiration. The Tyndall effect that occurs due to a large injection amount can be solved by reducing the amount.
To prevent the Tyndall effect and transparent effect, filler should be injected in small amounts and injected deeply in high-risk areas. Colored fillers should not be used in these areas. Patients should be warned when such fillers are used in high risk areas.
1.10 Skin Marking
Skin marking is an extruded scar created by the filler injection due to extensive extension of the skin such as striae gravidarum. Less extension and excessive soothing like striae distensae appear when too much filler presses against superficial skin. If the pressure continues, a permanent scar may form. If the filler contains hyaluronic acid, hyaluronidase can be used for degradation.
1.11 Filler-Induced Hypersensitivity Inflammation and Granuloma
Granuloma is the permanent tissue change that occurs after repetitive tissue reactions in which it becomes hard and solid. There are many assumptive causes, including filler toxicity (especially cross-linking agents), osmolarity, pH imbalance, and hyaluronic acid impurities. These complications are visible in the cheek, chin, nose, and periocular areas.
Clinical symptoms include repetitive swelling, flushing, pain at the injection site, and spreading to the surrounding areas. This is likely to subside with anti inflammatory drug use, but since the tissue reactions continue, a hard solid nodule is likely to form that shows tenderness, compressive pain, or facial asymmetry.
A hypersensitivity reaction occurs when the patient is in an immunosuppressive state, tired, menstruating, or in an upper respiratory infection state. Thus, when a patient complains about repetitive swelling during such conditions, a filler-induced hypersensitivity reaction is likely the cause.
Cases have recently increased because of high doses of cross-linking agent or/and low-quality hyaluronic acid powder. We can assume that the manufacturing process would be a high potential cause of granuloma.
Granuloma is also likely to appear when a patient keeps touching the injected area because the injected filler is likely exposed to the adjacent tissue.
Hyaluronic Acid Filler and Hyaluronidase
Hyaluronic acid (HA) filler is a soft-tissue filler that is used in >80% of the market. Since the US Food and Drug Administration approved Restylane® (Q Med Company, Sweden) in 2003, HA fillers made of cross-linked HA have been commonly used because of their superior safety compared to other PMMA, PAAG, PCL, and PLLA fillers. Another advantage of HA fillers is that they can be dissolved in case of unexpected results, such as undesired outcomes or complications.
Clinicians should be educated about the fundamental manufacturing process and basic properties of hyaluronic acid fillers to prepare them for cases of complications. In this chapter, we will discuss the basic properties and associated complications of HA fillers and describe hyaluronidase, the most important drug used to treat such complications.
2.1 Hyaluronic Acid
HA is a disaccharide present in the skin, synovial fluid, and vitreous humor. Of the 12 g of HA present in a human being, 3 g is dissolved and synthesized daily.
HA has a disaccharide structure and usually exists as sodium hyaluronate. It is a glycosaminoglycan that has the same structure in animals and bacteria. Therefore, massive amount of HA manufactured from bacteria is harmless to human beings. That is why there is so many HA filler in the market.
Healthy human HA has a molecular weight of 5,000,000–10,000,000 Da; animal-based HA has a molecular weight of 4,000,000–6,000,000 Da, while the molecular weight of bacterial-based HA is 1,500,000–2,500,000 Da. However, the HA filler is made by cross-linking of these HA molecules to form a gel structure; therefore, the molecular weight does not differ much.
2.2 HA Filler
HA differs from HA filler. HA is sold in the amorphous form in the market. Since hyaluronidase naturally exists in the human body, HA should be cross-linked using a cross-linker to ensure its stability.
1,4-Butanediol diglycidyl ether (BDDE) is a popular cross-linker. Filler properties depend on factors like amount of BDDE used, time to react with BDDE, and reaction temperatures.
2.3 HA Filler Manufacturing Process
HA filler is made by mixing raw HA powder with a cross-linker, and each company uses different raw products, cross-linker concentrations, reaction times, temperature and manufacturing process.
Each manufacturer follows a different process. For example, some products are made by reaction at 50 °C for 2–3 hours, while other products are manufactured at 45 °C with a reaction time of 4 hours. The washing process is different for the products subjected to dialysis or those whose manufacture process involves a dehydration and reswelling process. Some products are mixed with non-cross-linked HA at the final stage.
A notable process is the degradation of raw HA by NaOH. At pH <8.0, the molecular structure of the HA carboxyl chain (-COOH) is altered since an ester bond may be formed; at pH >10.0 HA, the hydroxyl chain (-OH) may be involved in the formation of an ether linkage. This ether bond should be linked to BDDE, and a strong bond should be formed. However, since NaOH is alkaline, it may be harmful for the human body; thus, all surfaces should be washed after handling. Unlinked BDDE should also be washed out completely after the reaction. Thus, the washing process is extremely important The problem is that BDDE cannot be removed during the washing process. This could be a cause of chronic inflammation.
2.4 Properties of HA Fillers
There are hundreds of filler products in the market, each of which has a different manufacturing process, resulting in different properties of the fillers.
2.4.1 Biphasic Versus Monophasic
“Biphasic” and “monophasic” have been used frequently to differentiate among HA fillers. However, this categorization is based on misinterpretations of the term “phase” since it refers to differences among manufacturing processes. However, there are definite differences between Restylane, the most popular biphasic filler, and Juvederm, the most common monophasic filler. Biphasic fillers are known for their relatively high G′ (storage modulus) due to the HA particles within them. On the contrary, monophasic fillers have a relatively low G′ but higher cohesiveness. Between two fillers with the same cohesiveness, the stronger one with a higher G′ is used for nose or chin augmentations. However, products with a high G′ should have enough cohesiveness to hold the particles together to prevent migration. In contrast, since monophasic fillers have relatively high degrees of cohesiveness, they should be used in wide areas, such as the forehead. Manufacturers are now attempting to make fillers that have the advantages of both monophasic and biphasic fillers.
2.4.2 HA Concentration
Every filler has a declared HA concentration, the content of HA within 1 mL of filler. A product that contains 20 mg of HA is expressed as 20 mg/ mL; when there is a higher amount of HA, the filler would be long lasting and hard. However, since HA is likely to absorb surrounding water molecules, swelling might occur when the equilibrium is broken. Generally, 5.5 mg of HA in 1 mL of water reaches equilibrium, but since HA is cross-linked, there is no strict concentration.
HA is a naturally existing disaccharide in the human body and can pull large amount of water from adjacent tissues. Thus, equilibrium is important to preventing swelling. It is known that
5.5 mg HA in 1 mL reaches equilibrium, the solubility of HA in water is 5.5 mg/mL, but the most common cross-linker BDDE cannot provide a filler of sufficient hardness for lifting capacity. For this reason, the manufacturers make concentrations of 15 mg, 20 mg, 24 mg, and 33 mg; high concentrations would lead to initial swelling. Another cross-linker, divinyl sulfone, can provide sufficient hardness at low concentrations, but it is toxic and rarely used anymore.
2.4.3 Particle Size
When an HA filler contains small particles, it is best used in the dermal layer; in contrast, when it contains larger particles, it is best injected into the subcutaneous layer or beneath. Biphasic filler use is categorized by particle size. Every HA filler can be evaluated by a particle size analyzer that can estimate where it should be used.
2.4.4 Injection Force, Extrusion Force
Injection force is a parameter of how smooth the filler is injected. When the injection force (N) is high, relatively high power is needed to inject them; therefore, every fillers should be tested by needles with standard diameter. The company should specify that the product is easy to inject; therefore, they tend to use a larger diameter needle during testing. Thus, it is important to record the doctor’s own impressions instead of relying on just the company’s data.
This phenomenon might occur in biphasic filler because of the presence of uneven particles. Thus, the authors recommend the use of a 1–2 G larger-diameter needle than that recommended by the filler company.
When a small-diameter needle or cannula is used, the injection force should be high, but a high pressure can cause serious vascular compromises.
Using a small versus large diameter needle remains controversial. When using a small- diameter needle, there is a small chance of vessel puncture inside the vessel that can cause an embolism that moves to a farther location. When using a large diameter needle, there is a greater chance of vessel compromise, but it is impossible to locate inside the vessel, so the pressure is dis- tributed, and emboli cannot move to a farther location
This fact is very important for blindness or cerebral infarction caused by arterial regurgitation. There are some predisposing factors for blindness, including the needle end should be located inside the artery; the internal carotid artery branch should be used; the injection pressure should be higher than the arterial pressure for arterial regurgitation; and the filler amount should be greater than the arterial volume to cover the central retinal artery.
The use of a small diameter needle is affected by factors 1 and 3 listed above. A small diameter needle can enter the artery, and its pressure should be higher; for these reasons, we eliminated two predisposing factors by using a larger-diameter needle.
2.4.5 Cross-Linking Ratio, Degree of Modification (MOD)
As described previously, cross-linked HA filler consists of HA and BDDE. The use of a larger amount of BDDE in the manufacturing process can provide long lasting harder fillers. Thus, cross-linking ideally connects the bilateral sides of HA. However, there are some pendent types in which the cross-linker is attached to only one side of the HA. Also called the dangling type, it is useless for HA fillers.
Degree of modification, a parameter of both cross-linked and pendent HA, can be calculated by nuclear magnetic resonance (NMR). However, since MOD consists of both molecules, we should detect each separately. Each molecules can be detected by size exclusion chromatography com- bined with mass spectrometry (SEC-MS). This machine can be used to separately calculate cross-linked MOD (cMOD) and pendent MOD (pMOD). Thus, in cases of high MOD and high pMOD, the filler does not contain large amounts of cross-linked HA, while many complications might occur due to pendent HA. For example, Restylane has a relatively low reported MOD of 0.8 but has a similar cMOD to those of other products, showing that it is a relatively low pendent type. Recently, the pendent type has been one of the potent possible causes of filler-induced hypersensitivity, so many investigators are analyzing the use of NMR and SEC-MS.
2.4.6 Rheology
Rheology is an objective method to evaluate filler properties. Rheology is also the study of the flow such as viscosity, elasticity, plasticity, thixotropy and cohesiveness. Filler rheology elasticity and viscosity and cohesiveness are quite important and will be discussed later. Plasticity refers to the propensity of a solid material to undergo permanent deformation under a load, i.e., stress over elasticity. Thixotropy is the property exhibited by certain compounds that are liquid or have low viscosity when agitated or stirred but set slightly when standing still. For example, filler should be in the gel state in the syringe, become a liquid when injected through a needle, and assume a solid state inside human beings. Unfortunately, fillers remain in the liquid state after injection.
Various stresses are applied in humans when filler is injected; using a rheometer, we can estimate filler properties.
These are the relative important parameters of filler rheology:
1. G′: Elastic modulus, storage modulus, resistance to deformation
The filler deformation parameter is affected by external stress; when G′ is high, low deforma- tion occurs. This is not an exact parameter of hardness but is closely related. Fillers with higher G′ values will recover their shape better. Biphasic fillers usually have a relatively high G′.
2. G′′: Viscous modulus, loss modulus
The parameter of filler dissipated energy during shear stress due to friction differs from com- plex viscosity. Fillers with a high G′′ tend to lose energy and become liquid.
3. Complex viscosity
The parameter of a filler’s ability to resist flow indicates the filler’s thickness and is very much related to injection force.
4. Tan δ: tangent delta
This parameter is calculated as follows: G′/G′′. This parameter indicates whether a filler is likely to be solid or liquid. A value of tan δ > 1 indicates that it is likely to be a liquid.
5. Phase angle
This parameter involves the transformation of tan δ to an angle. When tan δ = 1, then the phase angle is 90°.
6. Elasticity: G*
This parameter of filler hardness is calculated by 100 × G′/(G′ + G′′). It is a percentage of stored energy divided by total energy. For example, if the filler is soft, total energy might be high, but energy loss is also high, meaning that, after the injection of soft filler into the skin, it will be easily deformed by skin compression and has low elasticity.
The rheometric parameters are not always the same; rather, they vary according to plate size, temperature, and frequency. Thus, a G′ of 500 does not specifically mean anything; rather, it is used for comparison only.
2.4.7 Cohesiveness
Cohesiveness is not a proper rheological term, but it is a very important parameter determining the filler properties. Unfortunately, it is not objectively calculated, and multiple methods are required to obtain objective data. A parameter of rheometer, referred to as tack data, serves as a cohesiveness index or indicator of diffusion capacity when injected into saline. Cohesiveness is important because of filler migration and molding procedures. The injected filler should aggregate each other to resist compressive forces.
Manufacturers recommend that some fillers be used in the subcutaneous layer, but some rheological data shows the filler might migrate when injected into the nose or chin because it has insufficient cohesiveness or storage modulus. Thus, it is important to determine filler properties and decide which one is suitable for use.
2.5 Hyaluronidase
HA fillers are used in >80% of the market because they can be degraded by hyaluronidase in cases of complications. Hyaluronidase is classified depending on whether the enzyme is obtained from animal testicles, leeches, or bacteria; hyaluronidase available in the market is usually made from bacterial components. The product is usually made of ovine or bovine testicle or human recombinant DNA and is used for hypodermolysis by dissolving normal HA but is also used to dissolve HA fillers (off label use). Hyaluronidase breaks bonds between N-acetylglucosamine C1 and glucuronic acid C4.
There are more than 20 products in the market; some are amorphous (Liporase®), while others are liquid (Hylex®). The product Vitrase® in the United States is made from ovine testicles and is 200 USP. Hylenex® is a human recombinant DNA product that is made with genetic manipulation of Chinese hamster ovarian cells and it is 150 USP. Hylenex is available at 150 USP and 200 USP in the United States, and 3–4 bottles are used to treat complications such as skin necrosis. However, in some countries like Korea or China, products at 1500 IU are available, so one bottle might be sufficient for the same treatment. (One International Unit [IU] = 1 USP.)
A skin test is recommended before the use of hyaluronidase. Although very rare, an immunologic reaction can occur since the product is made of animal origin. Its liquid form is also made from animal testicles and Vitrase® also can induce allergic reactions. Hylenex® might induce an allergic reaction because it contains albumin.
Dose: To dissolve overcorrected or unpleasant results of HA filler injection, the proper dose must be determined. One study described that to dissolve 0.2 mL of Restylane®, 10–30 IU of hyaluronidase is needed, but this is very much dependent on its manufacturing process. Each filler contains different amounts of cross-linking agents and was cross-linked for different amounts of time. An enzyme degradation test is used to calculate the time needed to dissolve HA filler. Every filler has a different degradation time. In the case of 1500 IU hyaluronidase, the use of a very small amount is recommended to dissolve unpleasant results. However, in cases of severe complications such as skin necrosis, a high dose is recommended to completely degrade HA filler. Multiple injections might aggravate predamaged skin, and since underdosing can result in leftover filler, an overdose injection is recommended.
It is recommended to use of 750 IU to degrade 1 mL of HA filler. This is generally an overdose, but it is important to completely degrade the filler. If not dissolved by this amount, the area assumed to be the filler could actually be a granuloma.
Some patients might ask to dissolve just part of the injected filler, but it is better to dissolve all the filler and reinject new filler. Since it is impossible to control the amount of degraded filler and the remaining amount of filler by injection of hyaluronidase, it is difficult to fulfill this patient’s request. When an insufficient dose of hyaluronidase is injected, another dose should be injected; if an overdose is injected, the filler should be reinjected.
Allergy: A skin test is recommended but not usually performed before the injection. Most products have the possibility of causing immunologic reactions, and the literature described incidences of urticaria and angioedema of <0.1%. Symptoms are severe swelling in the injected area <2 hours after injection. Thus, patients must be warned of the possibility prior to injection.
Treatments include oral antihistamines and corticosteroids.
Filler-Induced Hypersensitivity Inflammation and Granuloma
Hyaluronic acid filler is retained inside the human body for at least 1 year. Compared to drugs that are absorbed immediately, filler takes a significant amount of time to degrade. During this time, the filler may attack the human immune system and cause serious complications. Therefore, the filler should be manufactured aseptically, and new fillers should be assessed carefully for adverse effects. Many criteria for product licensure depend on laboratory data regarding any unexpected complications of a filler inside the human body.
The most common cause of chronic complications might be filler-induced hypersensitivity inflammation and filler-induced granuloma. Filler induced hypersensitivity inflammation occurs periodically and manifests as mild swelling to severe edema. This symptom is usually relieved by anti inflammatory drugs, which are used to treat it. However, repeated hypersensitivity tends to result in filler-induced granuloma, which usually requires surgical treatment. Thus, whenever this kind of symptom occurs, doctors should prevent granuloma formation by removing the filler at an early stage.
3.1 Filler-Induced Hypersensitivity Inflammation
Filler-induced hypersensitivity inflammation is also called repeated tissue reaction, immune reaction and delayed swelling.
3.1.1 Pathophysiology
Filler-induced hypersensitivity is considered a type IV hypersensitivity. The human immune system treats the filler as an antigen, thus activating macrophages and T lymphocytes to aggregate macrophages in the area of inflammation. This inflammation manifests as swelling and pain 2–3 weeks after the filler injection, and chronic inflammation can lead to granuloma formulation. The pathophysiology of this phenomenon is not clear; however, multiple suggested causes include filler toxicity, impurities, osmolarity, pH imbalances and endotoxins.
Hyaluronic acid filler is composed of hyaluronic acid mixed with a crosslinker (usually 1,4-butanediol diglycidyl ether [BDDE]). Multiple possible causes of filler-induced hyper- sensitivity include:
1. Raw hyaluronic acid: Hyaluronic acid is usually produced from bacterial hyaluronic acid and large amount of hyaluronic acid power is commonly sold. Among the bacteria, the Streptococcus species are used that may contain bacterial protein, DNA and endotoxin.
2. Hyaluronic acid is usually dissolved using a highly alkaline solution such as NaOH during the manufacturing process. The disaccharide product hyaluronic acid could be dissolved to a monosaccharide, and its by-product might induce an undesirable reaction in the human body. Moreover, the last step in the manufacturing process, washing, may not eliminate all of the NaOH solution.
3. Crosslinking process: Raw hyaluronic acid is converted to a long-duration hyaluronic acid filler by a crosslinking process using BDDE. The problem is some of this cross- linker links to just one side of the hyaluronic acid, creating a pendant type crosslinker.
When the washing process is properly done, the free and native types are washed out. However, the pendant type remains, making it a highly suspected cause of the filler-induced hypersensitivity reaction. Additionally, by products as a result of BDDE metabolism could cause some irritation, raising the possibility of human immune reactions.
Even when the products are purchased from the same company, products with a relatively high concentration of hyaluronic acid are associated with a higher incidence of filler-induced hypersensitivity. For this reason, BDDE is an important hypersensitivity related factor. Cases in which a large amount of filler is injected or multiple injections are performed show a higher incidence of this complication.
Raw hyaluronic acid is classified based on its usage as ingestion, cosmetic, and medical products; the latter is divided into injection and ophthalmologic products. Generally speaking, when the grade is higher, the cost is higher. Thus, a low cost product generally has more impurities and induces greater hypersensitivity. Therefore, if the product is low cost, the possible associated complications should be considered.
From the injector’s perspective, multiple causes are suspected. The use of a large amount of filler exposes the human body to more foreign bodies and might increase the incidence of hyper- sensitivity. Multiple injections, a large amount of filler injection, and the use of multiple kinds of products can cause greater hypersensitivity. Injections made into multiple layers can expose more surfaces to foreign bodies and increase the risk of inflammation. Few doctors propose injecting filler using insulin syringes, but this can change the physical properties of fillers and cause greater degrees of inflammation. This method can also increase the risk of infection. A highly molding procedure is also not advisable.
From the patient’s perspective, stimulation should be avoided in the injected area as much as possible. The patient’s immunologic state is very important, as a depressed immunologic state, such as the common cold or a highly stressed state, can increase the incidence of hypersensitivity.
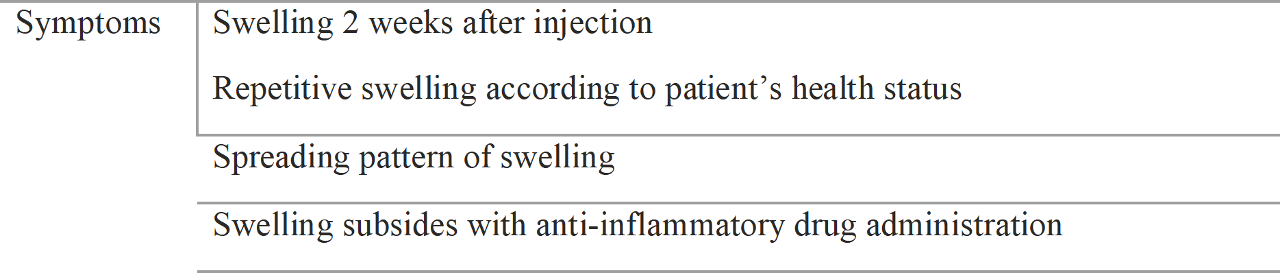
3.1.2 Symptoms
Swelling is the most common symptom of a hypersensitivity reaction. More severe symptoms include tenderness, pain, and fever. The symptom presentation starts within 2 weeks after injection as the human body reacts to the foreign body, usually in one region and spreading to adjacent regions.
Symptoms are usually associated with the patient’s health status. Common cold, menstrual periods, alcohol intake and other stresses might decrease the patient’s immunologic status and induce swelling. Initially, subclinical swelling and other symptoms that might go unnoticed occur but the symptoms usually become severe. Clinicians should consider filler induced hypersensitivity after noting the following symptoms
The most common sites are the cheek, chin, and premaxillary region, followed by the lip, nose, periocular area, and forehead. Although the cheek, chin, and premaxillary regions receive relatively large amount of fillers, swelling is easily detected in these areas.
3.1.3 Differential Diagnosis
The use of filler injections has increased, and patients tend to complain of various associated symptoms. Thus, it is essential to differentiate between filler induced hypersensitivity and natural swelling. Filler induced hypersensitivity tends to develop at least 2 weeks post injection. They also usually develop unilaterally at the nasojugal groove area and cheek and spread to other locations.
Filler-induced hypersensitivity tends to develop according to a patient’s immunologic status, such as during menstrual periods, common cold, or in cases of a depressed immunologic status. Filler-induced hypersensitivity usually develops unilaterally and subsides with anti-inflammatory drug use, whereas false hyper- sensitivity swelling usually does not subside. When filler-induced hypersensitivity continues, a granuloma develops and multiple nodules are detectable at the lesion. A granuloma can be detected by ultrasound.
3.1.4 Treatment
Anti-inflammatory drugs are generally effective in cases of mild swelling or tenderness. Steroids can improve symptoms but are not essentially needed. The question is whether symptom improvement cures filler-induced hypersensitivity. Once filler has induced hypersensitivity, it will act as a foreign body antigen, so all hyaluronic acid filler may require elimination by hyaluronidase. The best timing for hyaluronidase administration is when the hypersensitivity first occurs, but it is not easy to convince the patient of the need to dissolve the filler. Thus, it is recommended that the clinician tells the patient about possible recurrence of hypersensitivity and the need to dissolve the fillers.
When dissolving the filler, it is recommended that all fillers be injected at the same time. If only part of the filler is dissolved, the remnant filler might induce another hypersensitivity reaction.
Table 3.2 Symptoms of filler-induced hypersensitivity
Anti-inflammatory drugs are generally effective in cases of mild swelling or tenderness. Steroids can improve symptoms but are not essentially needed. The question is whether symptom improvement cures filler-induced hypersensitivity. Once filler has induced hypersensitivity, it will act as a foreign body antigen, so all hyaluronic acid filler may require elimination by hyaluronidase. The best timing for hyaluronidase administration is when the hypersensitivity first occurs, but it is not easy to convince the patient of the need to dissolve the filler. Thus, it is recommended that the clinician tells the patient about possible recurrence of hypersensitivity and the need to dissolve the fillers.
When dissolving the filler, it is recommended that all fillers be injected at the same time. If only part of the filler is dissolved, the remnant filler might induce another hypersensitivity reaction.
Table 3.2 Symptoms of filler-induced hypersensitivity
The dosage of the dissolving compound should be higher than the dosage of the filler to ensure dissolution of all the fillers. We prefer to use half a bottle at once (750 IU). This is generally a high dose, but the dosage should be enough to dissolve the filler; if the filler does not dissolve, it might not be hyaluronic acid.
When symptoms recur even after hyaluronidase injection, the clinician should check the granuloma or nodule using an ultrasound device and inject a higher dose of hyaluronidase. When symptoms recur after the second hyaluronidase injection, computed tomography or magnetic resonance imaging should be used to detect occult granuloma and/or the patient should be transferred to special filler complication clinics.
3.2 Filler Granuloma
The incidence of filler induced granuloma has increased recently; therefore, precise diagnosis and treatment are required.
3.2.1 Pathophysiology
Repetitive filler-induced hypersensitivity inflammation would induce a granuloma. Repetitive inflammation causes a filler capsule of increasing size. The filler is recognized as a foreign body that induces inflammation and hypersensitivity. Macrophages emerge to phagocytize foreign bodies but fail and then develop into multinucleated giant cells. Fibroblasts are activated by the macrophages, and a fibrous capsule develops as a hard lump. This inflammatory process is worsened by any infection, presence of biofilm, and impaired immunologic status. A granuloma develops by this process over a period of at least 3 months. A small nodule develops prior to a hard, tender granuloma. A granuloma can be located at the nose, forehead, anterior malar area, cheek, chin, and lips and is related to the incidence of filler injection and closely related to filler induced hypersensitivity.
The first symptom is a nodule, so it is helpful to diagnose granuloma by accurate history taking and ultrasonography.
A granuloma can also be induced by the use of fillers such as polyacrylamide gel or foreign body products such as silicone.
3.2.2 Classifications
Granulomas can be classified as cystic, nodular, sclerosing, or infiltrating depending on their final shape. A cystic type granuloma is usually induced by hyaluronic acid filler or hyaluronic acid filler located at a cyst. A nodular-type granuloma usually contains multiple nodules and is induced by hyaluronic acid filler or particle fillers such as calcium hydroxyapatite filler, polycaprolactone filler, and polylactic acid filler.
A sclerosing-type granuloma is usually seen after the injection of the polymethyl methacrylate filler or a foreign body filler such as silicone gel or paraffin. This type of granuloma can also be seen after a long-term infection or hypersensitivity induced by a hyaluronic acid filler injection. It can also be seen after the inappropriate repetitive treatment of a previous granuloma.
An infiltrating-type granuloma manifests as a huge lump with swelling that consists of filler and inflammatory cells. It usually develops after exposure to foreign bodies or permanent fillers.
3.2.3 Treatments
Multiple treatments are proposed. Firstly, hyaluronidase after hyaluronic acid filler is injected.
Hyaluronidase should be injected into the capsule of a cyst or nodule, usually at a high dose (1500 IU of hyaluronidase mixed with 2 mL of normal saline). However, a granuloma is rarely completely treated by hyaluronidase injection alone since it likely exists as multiple rather than a single capsule. Thus, removal of the filler as well as surgical removal of the capsule is recommended. Surgical excision is the best method to remove all of the fillers and capsules, but surgical sequelae such as scarring and depressive wounds can develop, for which we prefer to employ negative pressure suction.
Laser-assisted dissolving can be useful, but the best method is the surgeon palpating the granuloma during negative pressure suction.
A steroid injection is sometimes performed, but it can cause the development of depressive wounds. When using hyaluronidase, the use of two or three injections is recommended; if there is no response, a surgical procedure should be performed.
Danger Zones of Filler Injections
Two distinct phenomena can occur when doctors inject filler for the first time. First, the doctor feels that the procedure is very easy and features an immediate response without any danger. Second, the doctor feels absolute fear about where to inject and how much filler to use. Both attitudes occur because of a lack of knowledge.
Filler injection is an easy but potentially dangerous procedure. However, it is not difficult to learn, so safety can be ensured with basic knowledge.
4.1 Facial Danger Zones
Facial danger zones during filler injection are quite different from those during surgery. Surgery is basically a “destroying procedure,” so danger zones include areas containing nerves and vessels. In comparison, fillers are basically used to fill an area, so inflated tissue properties are very important. Thus, we must consider the new concept of the facial danger zone in contrast to the surgical danger zone.
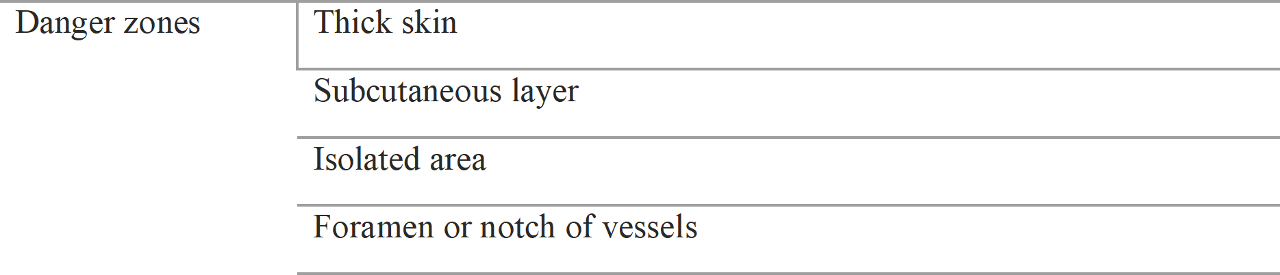
Location of danger zone during filler injection is shown in Table 4.1
Table 4.1 Danger zones
4.1.1 Thick Skin Area
Thick skin is hard and tough, so when the filler is injected, high resistance is encountered. Vessels between injected fillers and thick skin tend to increase the risk of necrosis compared to those in thin skin.
Studies have shown that the nasal tip, glabella, cheeks, and chin have relatively thick skins and that the most noticeable areas are the glabella and nasal tip. These two areas are most commonly treated with a filler, which tends to be injected superficially, and carry a higher risk of compression.
4.1.2 Subcutaneous Layer
The arteries of the face run either from the internal carotid artery and run through the facial foramen or from the facial artery from the external carotid artery branches. They usually run near the bone or through the foramen and run gradually to the superficial subcutaneous layers. There is a high risk of vessel injury when the filler is injected superficially because most vessels already run superficially.
The subcutaneous arteries have smaller diameters than the deep arteries, which increases the risk of ischemic necrosis when high-pressure filler is injected near the subcutaneous tissues. The risk is also increased when the filler is injected into thick skin.
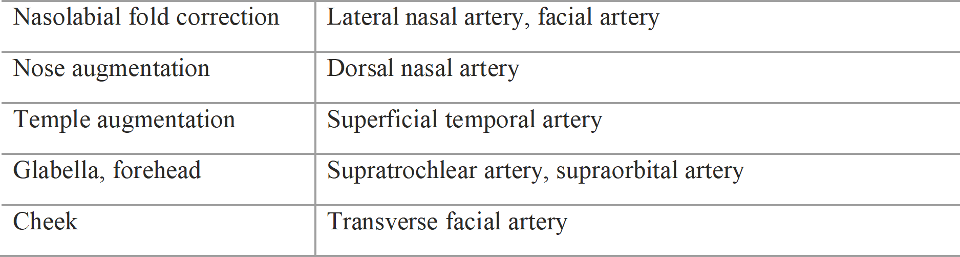
Clinically important arteries include the:
1.Supraorbital artery
2. Supratrochlear artery
3. Lateral nasal branch of the facial artery
4. Dorsal nasal artery
4.1.2.1 The Supraorbital Artery
The supraorbital artery is a branch of the ophthalmic artery from the internal carotid artery that runs through the supraorbital notch or foramen and deeply under the frontalis muscles and/or runs superficially to create an anastomosis with the supratrochlear and superficial temporal arteries
The deep branch of the supraorbital artery might be located 12 mm above the orbital rim, so it should be approached very carefully. It can continue running deeply until 16–42 mm; therefore, the filler should be very carefully injected into the supraperiosteal layer. The skin is usually elevated at the squared area because of the corrugator muscle
4.1.2.2 The Supratrochlear Artery
The supratrochlear artery is a branch of the ophthalmic artery along with the supraorbital artery. The internal carotid artery branches from the ophthalmic and the supratrochlear artery posterior to the trochlear, perforates the medial orbital septum, and runs to the glabellar area. It tends to create an anastomosis with the contralateral supratrochlear artery.
After exiting the orbit, it runs superficially, so skin necrosis often occurs after injections are made to correct glabellar frown lines. This area is relatively thick, so it involves a higher risk of compression.
corrugator muscle
4.1.2.3 The Lateral Nasal Artery
The lateral nasal artery is a branch of the facial artery at the level of the alar crease. The facial artery tends to run deeper than the zygomaticus major and zygomaticus minor muscles and superficially to the levator labii superioris and levator labii superioris alaeque nasi muscles. Thus, the lateral nasal artery is located in the subcutaneous layer
The lateral nasal artery is susceptible to injury during filler injection for nasolabial fold correction because it tends to run superficial to the sub- cutaneous layer between the nasolabial fold and the upper part of the nasolabial fold, called the premaxillary or infraorbital region. When injections are made into the subcutaneous layer in this area, there is a high possibility of damaging this artery.
When the facial artery is constricted due to an infraorbital nerve block by epinephrine anesthesia, the superior labial, lateral nasal, and dorsal nasal arteries could be constricted simultaneously. Therefore, it is likely to affect the adjacent vessels because they create an anastomosis.
The lateral nasal artery runs subcutaneously and is commonly damaged. Most doctors do not augment their work right away, but over time, they try to perfect their results by injecting into this area and compromise the vessels.
4.1.2.4 The Dorsal Nasal Artery
The dorsal nasal artery is the ophthalmic artery branch from the internal carotid artery. It supplies the nose after perforating above the medial palpebral ligament at the orbit and then creates an anastomosis with the contralateral dorsal nasal artery and the lateral nasal artery. This vessel also runs through the subcutaneous layer; thus, a superficial injection may cause vascular compromise.
Four arteries have been described. The supraorbital, supratrochlear, and dorsal nasal arteries arise from the internal carotid artery, while the lateral nasal artery arises from the external carotid artery. The three vessels arising from the internal carotid artery are important because their compromise could cause the most tragic filler complication, blindness induced by retrograde filler injection.
The lateral nasal and angular arteries also create an anastomosis with the dorsal nasal artery, so any filler injections made near the internal carotid artery should be done very carefully. These arteries tend to run through the subcutaneous layer, so avoid making injections into the superficial layer or use a large-diameter needle such as a 23G to prevent a high pressure injection. If a high pressure filler injection is made and filler is injected retrogradely, serious complications such as blindness may occur.
4.1.2 Isolated Area
Some regions have different skin properties and anatomical structures. One example of such a region is the nasal tip, which is composed of thicker skin than the nasal dorsum and has a unique structure in which the subcutaneous tissue is tightly bonded with the SMAS layers. This area is important because pressure that is introduced with injections cannot be diffused, which increases the risk of necrosis
In contrast, the dorsum area is at relatively low risk because its skin is thinner and loosely connected between subcutaneous tissues and the SMAS. When making injections into the nasal tip area, it is very important to inject 70% of the maximum amount to decrease the pressure. Especially when injecting fillers that tend to cause swelling, such as calcium hydroxyapatite filler and polycaprolactone filler, it is important to consider injecting only 60% of the maximum amount.
Regarding these properties, when injecting filler into the nasal tip area, clinicians should follow up with the patient the next day to check for pain, color changes, and swelling; if problems are noted, early decompression is very important.
4.1.3 Foramen
A foramen is a hole through which vessels perforate the bone. Important vessels include the supraorbital artery from the supraorbital foramen and the infraorbital artery from the infraorbital foramen.
The regions in which vessels perforate should be approached very carefully because vessels can be damaged if injections are made nearby. The danger is increased because the foramen holds the vessel, similar to holding a vessel in one’s hand. This area is also important when a local anesthesia is injected because nerves can be damaged as well.
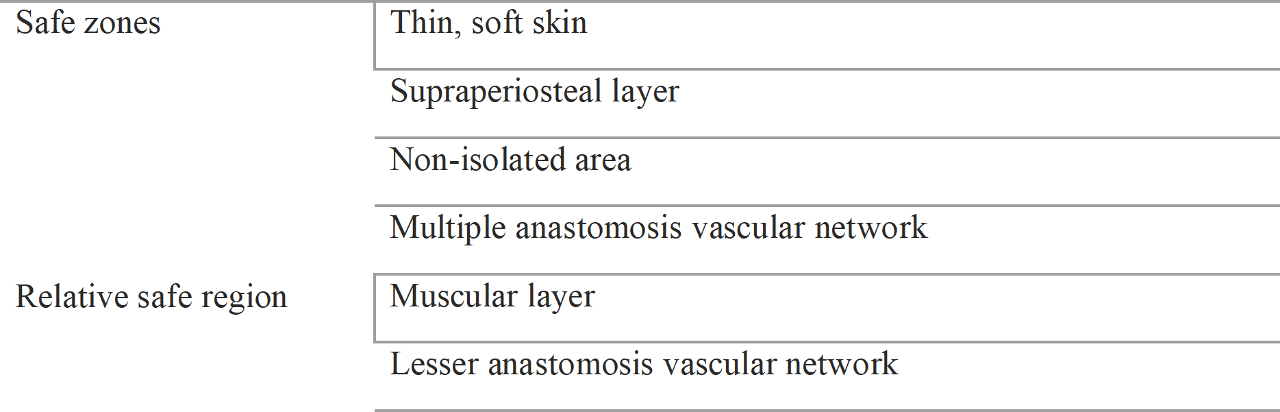
4.2 Safe Zones
Safe zones are opposites of danger zones. Smooth and thin skins can disperse pressure by allowing skin surfaces to expand.
Just above periosteal or perichondrial layer is an avascular plane, a target layer for surgery. For the same reason, it is safe for filler injection.
The nasal tip is an isolated high-risk region; in contrast, the nasal dorsum area can disperse pres- sure when filler is injected, making it relatively safe. When one pinches and moves the nasal tip skin and the nasal dorsum skin, the differences become clear as the nasal tip feels like a lump and the nasal dorsum glides smoothly. This phenomenon occurs because of differences in the tight bonding between the subcutaneous tissue and the SMAS.
Locations where multiple vessels create an anastomosis could be safe places because of col- lateral circulation. These places are the lips and eyelids, which are at relatively lower risk of vascular compromise.
Table 4.3 Safe filler injection zones
The muscular layer is also known to be relatively safe because it can disperse the pressure, but it has many vessels, so it is not completely safe.
4.3.1 Glabella
The glabella region contains thick skin, and the supratrochlear artery (arising from the internal carotid artery) is located within the subcutaneous layer. Therefore, localized skin necrosis due to thick skin or blindness and cerebral infarction due to embolism can occur.
To prevent these complications, minimal amounts of filler should be injected into a supra- trochlear artery location if possible. .
When injecting into this area, the needle should be advanced and aspirated, and the injection should be made gently with minimal pressure using a large-diameter needle such as a 23G. The first injection should be made into the preperiosteal layer to create the foundation for the whole procedure, while the last injection should be made into the subcutaneous layer. Injections made into the subcutaneous layer require more attention. When injecting into the subcutaneous layer, clinicians must advance and aspirate the needle to check for blood and then make the injection carefully to ensure low skin tension.
Hyaluronic acid fillers tend to cause water retention and expand, so 70% of the maximum amount should be injected.
4.3.2 The Forehead
The supraorbital artery also arises from the internal carotid artery, so filler injected into the vessel could cause blindness or cerebral infarction.
The supraorbital artery has a superficial branch and a deep branch. It runs at subcutaneous layer, and it is safer to inject filler at the supraperiosteal layer. However, even in supraperiosteal injection, it is not completely safe because as in type III deep branch, it could run into the supraperiosteal layer until 16–42 mm from the supraorbital rim and until 12 mm at supraperiosteal layer. Therefore, injections should not be given below 12 mm and should be done carefully for 12–42 mm.
4.3.3 The Nasal Root
The nasal root region is supplied by the dorsal nasal artery, and this artery arises from the internal carotid artery, which leads to the risk of blindness and cerebral infarction.
Recently, there have been cases of blindness during nasal augmentation by filler injections because of embolism of dorsal nasal artery and regurgitation to ophthalmic artery. One of the common procedures for filler injection in Asian is nasal augmentation, and it is very important to concern about embolism. Nasal root area is not an isolated region com- pared to the nasal tip, and the skin is thinner than the skin of nasal tip. Also, the skin is not tightly connected with the SMAS layer. Therefore, the nasal root is a relatively safer region for skin necrosis by compression. However, there is a higher tendency of injection to the subcutaneous region to make sharper shape nose, and this phenomena could increase the risk of embolism.
Moreover, by using along cannula, this risk has been increased.
To prevent this complication, it is important to use a large-diameter cannula or needle (e.g., 23G), making it easier to deliver the needle tip to the desired layer. However, many injectors like to use cannula. A cannula has a blunt tip and is easy to localize within the subcutaneous layer, which features less resistance. Thus, when a cannula is used, it is important to control the cannula tip. A cannula larger than 21G should be used to more easily control its direction. When the cannula tip has reached the nasal bone and septal cartilage junction, the cannula tip was located in the supra- periosteal layer, meaning that the needle can scratch the bone prior to the injection.
4.3.4 Nasal Tip
The nasal tip typically contains thick skin that is tightly attached to the SMAS. It is a solitary region, so when a large amount of filler is injected into it, the pressure cannot be dispersed and skin necrosis may occur. In particular, the subcutaneous layer is not soft, so it is vulnerable to skin necrosis.
The injector may tend to augment the sharp nasal tip and tend to inject filler into the subcuta- neous layer, where the lateral nasal artery is susceptible to injury. Considering maximal tension as 100%, an amount lower than 70% should be injected. The other 30% should remain to account for injection swelling and water retention. Injections between the two alar cartilages are safe.
When injecting into the nasal tip, it is important not to create a wide tip. The authors like to use a 23G needle when augmenting only the nasal tip.
4.3.5 Ala Nasi (Wing of the Nose)
The ala nasi is the place more rigid than the nasal tip area, so filler injections into this region might cause localized necrosis. The skin is so rigid that even temporarily corrected grooves might be visible again within 2 weeks after treatment.
4.3.6 Infraorbital Foramen
The use of filler for premaxillary augmentation is increasing nowadays. It is usually safe to inject filler into the suborbicularis oculi fat (SOOF) layer.
When injecting filler into the deepest part, one should avoid the infraorbital foramen, where the infraorbital nerve and vessel perforate. This is likely to hold vessels and nerves down one side, so puncture here is highly dangerous. The infraorbital foramen is directed downward and one step from the infraorbital rim, so it is safer to make the injection in the downward direction rather than the upward direction.
4.3.7 Nasolabial Fold
The nasolabial fold is one of the most common filler injection places. Since many procedures are performed, many complications also occur. The most noticeable vessel is the lateral nasal artery, which runs from the facial artery branch at the alar crease.
The facial artery tends to run deep to the zygomaticus major and minor muscles and run superficially to the levator labii superioris and levator labii superioris alaeque nasi muscles. The lateral nasal artery is located in the subcutaneous layer. Of course, not every human has the same arterial system, but the subcutaneous layer should be considered.
This vessel tends to be damaged during nasolabial fold correction. The lateral nasal artery crosses from the premaxillary or infraorbital region to the upper part of the nasolabial fold. Thus, injections into this region tend to damage the vessel. The lateral nasal artery tends to be injured in the area indicated by the arrow. Alar rim necrosis or nasal tip necrosis could occur, while blindness or cerebral infarction might occur due to dorsal nasal artery embolisms that connect with the angular artery. Such damage leads to alar rim necrosis or nasal tip necrosis, while blindness or cerebral infarction might occur due to dorsal nasal artery embolism.
Lateral nasal injuries tend to be made by experienced rather than beginner injectors since doctors with sufficient experience and confidence making filler injections tend to achieve better results and make injections into the subcutaneous layer where the lateral nasal artery is located.
Thus, when correcting a nasolabial fold, always remember the pathway of the lateral nasal artery.
4.3.8 Temple
The temple’s anatomy is quite complicated. We define the temple area from the superior temporal septum to the zygomatic arch, and it contains many different layers. From the outside, there is the skin, subcutaneous layer, superficial temporal fascia (temporoparietal fascia), innominate fascia and parotid temporal fascia, deep temporal fascia superficial layer, deep temporal fascia deep layer, temporalis muscle, and temporal bone. The layer should be compared with other facial regions
Theoretically, we can inject soft-tissue filler into four spaces: (1) subcutaneous layer; (2) space between the superficial temporal fascia and the deep temporal fascia; (3) space between the superficial layer and the deep layer of the deep temporal fascia; and (4) under the temporalis muscle
The first space is the subcutaneous layer, which is a superficial fat compartment that consists of the lateral temporal cheek compartment and the lateral orbital compartment. The superficial temporal vein and the sentinel vein are located in this layer. Veins can be detected in thin skin, but when torn, severe bruising might occur. When injected deeply, the superficial temporal artery or zygomatico orbital artery can also be damaged. We recently used near infrared technology to detect veins and avoid injuring them
The second space is that between the superficial temporal fascia and the deep temporal fascia. This area can be divided by the inferior temporal septum into the upper temporal compartment and the lower temporal compartment. The upper temporal compartment is a relatively safe region, and the lower temporal compartment should be considered the deep temporal vein perforating branch. To use a cannula, we must puncture the superficial temporal fascia and inject filler into this area. The deep temporal fascia is very hard, making it difficult to perforate by a cannula; in contrast, it is quite easy to inject into the upper temporal compartment.
The third space is that between the superficial layer and deep layer of the deep temporal fascia, which includes the temporal fat pad. The problem is that we cannot approach this area using a cannula because we must perforate the deep temporal fascia superficial layer, which is difficult to do with a cannula. When using a needle, we cannot estimate this layer. Also, if we inject a little deeper than the deep temporal fascia, we may encounter the muscle and buccal fat pad temporal extension, at which point the filler might migrate to the buccal portion.
The fourth space is that under the temporalis muscle. It is a relatively avascular space, but it might involve multiple problems: (1) Temporalis muscle is a mastication muscle that is firmly attached to the temporal bone, so filler should be injected inside the muscles, where it will be absorbed very quickly. (2) A large amount should be injected to lift both the fascia and the muscle, which requires more than 1–2 cc of filler. (3) The middle temporal vein, which runs horizontally at the temporal fossa under the superficial layer of the deep temporal fascia and is connected to the superficial temporal vein, should be avoided to prevent serious complications. The middle temporal vein is known to be located
23.5 mm (15.7–33.6 mm) above the jugale of the zygomatic arch and 18.5 mm (12.5–23.5 mm) above the zygion, one fingerbreadth above the zygomatic arch. (4) According to a recent paper, there was a case report of penetration of the temporal bone during a needle injection. It is difficult to perforate the deep temporal fascia using a cannula; thus, a needle should be used to make deep injections.
Skin Necrosis of Filler Injections
Skin necrosis after filler injection is quite a con- fusing experience for patients, while clinicians feel terrible having delivered what they assumed was a safe procedure. Consequences can even include permanent scarring.
Surprisingly, skin necrosis usually occurs when experienced rather than novice doctors perform filler injections since novice doctors tend to inject filler very carefully, while experienced doctors tend to increase the filler amount and inject it into multiple regions. Sometimes experienced doctors make the mistake of developing their own injection method without scientific evidence and considering filler injections an easy procedure. Thus, evidence based education about skin necrosis is needed.
5.1 Skin Necrosis Definition and Mechanism
5.1.1 Skin Necrosis Definition
Necrosis, defined as irreversible tissue damage followed by ischemic changes, occurs due to a break in the normal defense mechanism by ischemia-induced loss of tissue viability. In case of infection, extensive tissue can be destroyed by infectious necrosis.
Necrosis starts in the setting of a reduced vascular supply due to a direct embolism or compression by adjacent pressure. Filler injection induced necrosis usually develops due to increased pressure rather than the direct needle puncture of a vessel.
Risk factors of necrosis are as follows:
1.More superficial filler injection
2.Thicker skin
3.Harder skin
4.Tighter skin
5.Larger filler amount
6.Greater swelling
7.Small needle diameter
5.1.2 Mechanism
The vascular network becomes thinner and tighter as it approaches the dermal layer. The dermis is harder and tighter than the deeper sub- cutaneous layer, so superficial injections increase the risk of vascular compression. Pressure also increases when a larger amount of filler is injected or tissue swelling develops.
The most common misunderstanding is that using a smaller-diameter needle is safer. According to Bernoulli’s law, when a small diameter needle is used, the injection pressure should be higher. While using a small-diameter needle, the injector feels greater viscosity and injects the filler with greater force. A smaller needle tip is also more likely to puncture a vessel and create an embolism; therefore, such needles are more dangerous than larger-diameter needles
Sudden swelling during an injection is likely indicative of bleeding. Compression is com- monly used to treat such cases, but when filler is injected, compression could create an embolism and thus should not be done. Bleeding is a sign that the vessels are damaged and blood is extravasating. With compression, injected filler could be pushed into the vessel. When signs of bleeding are seen, it is best to stop the injection, remove the needle, and wait until the bleeding stops.
5.2 Classification of Skin Necrosis
Skin necrosis can be classified into localized and extended types. Localized necrosis develops at the injection site, whereas extended necrosis extends to the vascular territory. The most severe complications of extended necrosis are blindness and cerebral infarction.
5.3 Localized Skin Necrosis
The dermal plexus is a small vascular network system located at the dermis or hypodermis. When filler is injected into this area, it is likely to compress the vessels and lead to skin necrosis because the tissue is unable to disperse the pressure. Interruptions in the circulation can also cause blanching. This blanching ischemia finishes within 30 minutes, and the localized change leads to a dark pinkish appearance and development of a pustule within 48 hours. Thereafter, infectious necrosis is likely to develop.
Mild vascular compression showing a small pustule and pinkish color might not require treatment. However, when compression is severe, the skin’s color changes to a dark red wine color, and a pustule develops at each sebaceous gland. Pustules are likely connected to each other at the subcutaneous layer like underground water, and extensive infectious necrosis occurs. Tissue necrosis gradually develops and ultimately resolves as a depressive scar. If not treated properly, exudates changes to hard scabs that cover the necrotic surface. When a thick scab is formed, the infection progresses further, leading to further destruction of the subcutaneous tissue. An understanding of its pathophysiology is needed to determine the proper treatment
5.3 Localized Skin Necrosis
5.3.1.1 Decompression
Blanching after a filler injection should immediately be treated with decompression. When hyaluronic acid filler is injected, a high dose is needed to dissolve the filler. We prefer to inject one vial (1500 IU) of hyaluronidase mixed with 1–1.5 mL of normal saline and massage gently. There are no guidelines for hyaluronidase injections, but we prefer to use a high dose. If any filler remains undissolved, more hyaluronidase should be injected, but since the tissue is very friable due to ischemia, it is recommended that the remaining filler be dissolved at once. When permanent or calcium filler remains undissolved, it must be removed as soon as possible using negative pressure with large-diameter (18 G) needle aspiration. It is important to call the patient 1 day after injection to check for any color change or specific symptoms. A photograph of the patient should be checked if unusual progress is seen. Patients are likely to come to the clinic after the second day with complications because of pustules. Thus, if decompression is performed as soon as possible, patient status should be checked 1 day later.
Pustules usually develop within 2 days after the injection, so patients tend to visit the clinic themselves. Therefore, it is very important to check 1 day after the injection. Patients who tend to describe their symptoms as not severe should be evaluated using a photograph or in person.
5.3.1.2 Pustule Removal
Pustules likely develop if decompression is not performed or vessels are severely compressed within 48 hours. More aggressive treatment should be performed when pustules develop before 48 hours because such cases can progress to severe necrosis.
Pustules should be removed gently because the area including the nose and maxilla is con- sidered the danger triangle of the face due to venous communication between the facial vein and the cavernous sinus. A retrograde infection can spread to the brain, causing cavernous sinus thrombosis or meningitis.
The appropriate drainage of pustules can be encouraged by the use of oral medication.
Pustules are likely to spread within 48–72 hours, reduce 4 days after, and disappear within 6 days after the injection. Thus, pustule removal should be performed twice daily at 2–4 days after the injection. And it is important to prevent infection whenever possible and pre- vent depressed scar formation. After the acute infection stage, small pustules may appear but are easily cured.
5.3.1.3 Closed Wet Dressing
Necrotic tissue rapidly loses water and becomes covered by scabs. Pus under the thick scab tends to destroy the subcutaneous layer and create a depressed scar. Thus, a dressing should be applied to prevent scab formation. A wet dressing substitutes for the scab. We initially remove the pus and exudate, clean the wound, apply an antiseptic, and cover the wound with Vaseline gauze. The occlusive dressing is used until the pustules disappear. Hydrocolloid products are not recommended because they are best used when dressing changes are not required.
It is important that the skin not become detached during dressing changes, so we must be careful when removing the Vaseline gauze. Gentle removal and the use of a watery antiseptic are recommended. Even if the damaged skin is unviable, it is still useful as a biological dressing. Comparison of the removed skin and unremoved skin groups revealed that the unremoved group had much better results. Use of the Vaseline gauze dressing also minimizes skin defects.
5.3.1.4 Treatment After Acute Stage
After the acute stage, which should be treated as soon as possible, careful observation and education is needed to minimize sequelae. When providing aggressive and appropriate treatment, a complete cure can be accomplished without sequelae. Minor sequelae may include dermatitis or small pustules. Post-inflammatory hyperpigmentation occurs easily and is difficult to treat, so close observation and education are needed. Hyperpigmentation has no specific treatment other than UV protection for 6 months. Severe sequelae such as contracture, skin defect, and a depressive scar could occur when inappropriate treatment is provided and then require secondary treatment such as cell therapy.
5.3.1.5 Misunderstanding Treatment
Filler injections have been proposed as ground- breaking treatments, but many of them are not scientifically proven. Hyperbaric oxygen therapy has been proposed as a good treatment. However, problems occur when only hyperbaric oxygen therapy is used. We have multiple experiences with filler complications and bad prognosis in patients who were treated with only hyperbaric oxygen therapy. Hyperbaric oxygen therapy should be an adjuvant treatment. The most important treatments are pustule removal, decompression, and occlusive dressings
Another proposed treatment is stem cell therapy, which will be groundbreaking in many medical fields. However, it is not useful for treating acute ischemic necrosis. Stem the pustules have cleared.
A skin graft is useful for treating skin defects. However, a skin graft should never be performed for a nose defect because the nose has distinctly thick skin and a texture that differs from other areas. The use of careful dressings that preserve the tissue and promote reepithelialization could lead to better final results
In the treatment of necrotic tissue, occlusive dressings should be used instead of open dressings. Open dressings dry the wound and lead to scab formation. During the acute stage, hydrocolloid products should not be used because the wound exudate should be properly removed.
Many treatments have been suggested, but they should be considered adjuvant therapy to encourage wound healing.
5.4 Extended Necrosis
Extended necrosis progresses as arborization of the vessel territories. Extensive necrosis occurs because of large vessel involvement and divided into proximal necrosis, that near the injection site, or distal necrosis, that far from the injection site.
5.4.1 Proximal Necrosis
This phenomenon is seen when larger-diameter vessels are compressed or occluded, rather than small-diameter vessels such as those in the subdermal plexus. Necrosis occurring along the vessel pathway resembles arborization.
The most necrotized area is the vessel affected region, and it spreads along the vascular territory of the area of proximal necrosis. Severe compression or thrombosis at the traumatized vessel and necrosis at that region is the cause. Proximal necrosis is usually combined with distal necro- sis since thrombosis usually occurs. Thus, when extended necrosis is detected, the involved vessel must be identified and decompressed as soon as possible. If hyaluronic acid filler is used, a high concentration of hyaluronidase is recommended to dissolve it. If a calcium or permanent filler is used, a needle larger than 18G must be used for decom- pression. It is important to confirm which vessel is involved and then decompress it. The wound dressing should be the same as that used to treat localized necrosis.
5.4.2 Distant Necrosis
The needle or cannula tip puncturing the vessel or the injection of filler into a torn vessel creates an embolism; in the most severe cases, blindness or cerebral infarction may occur.
In cases of distant necrosis, the most severe necrosis develops at the distant region including emboli occluding the vessel. Reactive hyperemia is not seen in the occluded vessel. Blood supply is likely increased due to the distant partial occlusion. This phenomenon can be seen in large- diameter vessels such as the facial artery and/or the superficial temporal artery
Cases involving large filler embolisms are susceptible to proximal and distant necrosis. In this case, much more severe necrosis develops and needs more aggressive treatments.
In cases of distant necrosis, sufficient hyaluronidase should be injected into the filler- injected portion and the distant necrotic area.
However, if the filler was a permanent type, such as calcium filler or PCL filler, its removal is not recommended because it might harm unstable necrotic tissues; rather, it should be dressed.
To reduce this risk, we must use a needle larger than 23G and inject the filler gently with low pressure. Avoid injecting into the main vessel layer. When the vessel is ruptured, one should stop injecting filler and wait until the bleeding stops. It is possible that the filler could move into the ruptured vessel during compression to stop the bleeding.
Visual Complications of Filler Injections
Blindness is the most tragic complication of filler injections. The pathophysiology of blindness is known, but its prevention and treatment remain to be elucidated. Many studies have examined this problem.
The incidence of filler injection-induced blindness is increasing. I reviewed all reported cases and found 50 up to September 2018. The most common causes of the increasing incidence of blindness are the rapidly increasing number of filler injections and improper injection techniques. Many cases of blindness can be prevented if the operator uses proper injection technique.
6.1 Incidence of Ocular Complications
Studies on ocular complications usually include autologous fat grafts or unlicensed products; thus, I reviewed all ocular complications by fillers published to date and found 50 cases reported up to September 2018. These 50 cases did not include fat grafts, unknown fillers, or unlicensed products. We analyzed the products that are currently being used. The most common site of ocular complications involved injections at the nasal area, followed by the glabellar area. More than 70% of all ocular complications occur in these two regions.
Ninety percent of ocular complications occur after injections are made into the glabella, nose, forehead, and periocular region, areas that are supplied by the ophthalmic artery branches. This shows that the ophthalmic artery from internal carotid artery is the main pathway to blindness and we should be very careful when injecting filler into territories of the ophthalmic artery branches.
This new report is different from previous literature because the previous literature usually includes cases of fat graft. Fat grafts should be differentiated from filler injections because the fat graft procedure usually injects large amounts of filler and is much more invasive, disturbing more vessels. The incidence of visual complications by filler injection shows that more than 70% are from glabellar and nasal region injections. This shows that the temple area and mental regions are relatively susceptible to visual complications.
Among all cases reported in the literature through September 2018, 44% were in South Korea (Tables 6.1 and 6.2). Even though Korean doctors are performing a lot of filler injection procedure, it is unreasonable that almost half of all visual complications occur in Korea, and it is estimated that other countries do not report such a high incidence of this tragic complication. The interesting thing about this report is that 84% of all reports are from Korea, China, Taiwan, and Japan because of the relatively higher demand for nasal augmentation by filler injections in Asian countries. This shows that nasal augmentation carries a high risk of blindness.
6.2 Pathophysiology
The pathophysiology of filler-related visual disturbances is quite simple. Injected filler is at higher pressure than arterial pressure, and when any ophthalmic artery branch becomes occluded, visual complications occur. Fillers are injected against arterial pressure and regurgitate into the skull portion and then run to the ophthalmic artery and occlude nearby branches. Thus, the supratrochlear, supraorbital, and dorsal nasal arteries from internal carotid artery branches and the arteries that connect to the internal carotid artery branches such as the angular and the lateral nasal artery could be the cause of blindness. Inside the skull, the internal carotid artery branches to the ophthalmic, anterior cerebral, middle cerebral, posterior communicating, and anterior choroidal arteries.
The ophthalmic artery is the first branch of the internal carotid artery; when filler regurgitation occurs over this artery, it can cause brain infarction
The ophthalmic artery is the first branch of the internal carotid artery and anastomoses with the superficial temporal, angular, lateral nasal, and inferior orbital arteries, which arise from the external carotid artery. The most important vessels are the supratrochlear, supraorbital, and dorsal nasal arteries. The most severe visual disturbance involves occlusion of the central retinal artery. When filler regurgitates proximal to the central retinal artery, the posterior ciliary artery tends to occlude andchoroid ischemia occurs
If the filler is prevented from entering the ophthalmic artery branch, fortunately, it could avoid ocular complications, and skin necrosis might occur.
Blindness involves the following conditions:
-
Filler is injected into an artery that connects to the ophthalmic artery.
-
The entire needle end perforates the arterial lumen.
-
Filler is injected against arterial pressure.
-
Filler amount should be sufficient to fill the arterial lumen located from the entry point to the central retinal artery.
In the first condition of blindness, the filler is injected into an artery that anastomoses to the ophthalmic artery. The ophthalmic artery branches into the supraorbital, supratrochlear, and dorsal nasal arteries, in which regurgitation can easily occur that reaches the central retinal artery because they are directly connected to each other. On the other hand, arteries arising from the external carotid artery such as superficial temporal and lateral nasal arteries are also connected to the ophthalmic artery but have their own pathway, making regurgitation into the ophthalmic artery anastomosis less likely. Previous studies described blindness cases after injections into the temple area, but since we excluded fat graft cases, we encountered no cases of injection-induced blindness.
The angular and lateral nasal arteries are branches of the facial artery that arise from the external carotid artery. These two vessels are connected to the dorsal nasal artery by the internal carotid artery branch. When filler is injected into the angular and lateral nasal arteries, it tends to run in the forward direction; when it reaches the dorsal nasal artery, it should run in the opposite direction. This is the same mechanism by which the superficial temporal artery connects to the supraorbital artery; it should run in the opposite direction against arterial pressure to cause blindness.
Filler injected at the nasolabial fold runs in the forward direction at the angular artery and the lateral nasal artery; however, when it reaches the dorsal nasal artery, the flow should regurgitate against the pressure to enter to ophthalmic artery. However, we hypothesize that ocular complications in cases of nasolabial fold injection can occur for reasons including the following:
1.A large amount of filler is injected into the nasolabial fold that then enters the artery.
2.Nasolabial fold correction is performed much more commonly than temple augmentation.
3.Dorsal nasal arterial pressure is relatively low and regurgitation occurs easily.
Even when the filler is not injected directly into the dorsal nasal artery, injections into connected arteries such as the lateral nasal or angular arteries can easily cause regurgitation into the dorsal nasal artery. Thus, when injections are made into the nasolabial fold, caution should be taken to prevent entry into the dorsal nasal artery pathway.
The second condition of ocular complications is that the entire needle should fit within the arterial lumen. The important thing about this condition is that the injection pressure is transferred into the vessel and filler can migrate to areas with higher pressure. The use of a larger-diameter needle carries a higher risk of vessel injury but a decreased risk of the needle puncturing the vessel. Thus, in cases in which a vessel is punctured by a large-diameter needle, the pressure is distributed and cannot reach distant locations. A review of the literature revealed that the supratrochlear, supraorbital, and dorsal nasal arteries have approximate 1 mm diameters. A relatively large-diameter needle (23G) has a small outer diameter (0.64 mm) that can be inserted into a 1-mm- diameter artery. Many doctors like to use 27G needles, which have an outer diameter of 0.41 mm and can be easily be inserted into an artery.
The third condition of visual complications is that there should be enough injection pressure to overcome arterial pressure and frictional force of the vessel wall. To reach the central retinal artery, regurgitation into the ophthalmic artery is necessary, which requires a high-pressure injection. Clinically, a needle with a smaller diameter is required to create a high- pressure injection. High pressure is needed when injecting using a small-diameter needle than a large-diameter needle. Also, when a biphasic filler is injected, a small diameter needle could become occluded by large particles, so relatively higher pressure is needed. If occlusion is felt during the filler injection procedure, it is better to stop the injection and possibly change the needle.
The fourth condition is that a large amount of filler is needed to occlude a vessel from the entry point to the central retinal artery. Thus, when the entry point is far from the eye, there is a lower chance of blindness. It is also true that a small amount of filler could result in complications when injections are made at the nose, glabella, or periorbital area because the distance is short.
Regarding these conditions, the most important locations are the internal carotid artery branches, i.e., the supratrochlear, supraorbital, and dorsal nasal arteries are the most dangerous locations. Thus, clinicians should always monitor for the risk of blindness when performing filler injections to the nose, forehead, glabella, and periorbital areas.
6.3 Symptoms
Sudden severe pain is the most common symptom of blindness. Blurred vision, hemianopsia, decreased visual acuity, skin necrosis, and blepharoptosis could also occur. The symptoms are related to the arteries that are occluded by the regurgitation. When filler is injected into the supratrochlear, supraorbital, and dorsal nasal arteries and cannot reach the ophthalmic artery, only skin necrosis would occur. However, when more filler is regurgitated into the ophthalmic artery, PION, BRAO, CRAO, LPCAO, GPCAO and OAO might occur. Even when regurgitation into the internal carotid artery occurs, a brain infarction also might occur. Thus, the symptoms and skin necrosis patterns can indicate which artery has been affected.
Recovering from blindness is very difficult; in some recovery cases, the first symptom is partial visual loss. Thus, the prognosis is very much related with the first symptoms.
The most important thing might be when said symptoms occur. Many studies have suggested multiple treatments, but the pupillary light reflex must be checked first. This examination is not diagnostic; rather, it is the only tool that can screen for real ocular problems. If pupillary dilatation is present, one should consider using a retrobulbar hyaluronidase injection instead.
6.3 Treatments
6.3.1 Emergency Treatment
There is no definite treatment for ocular complications. Many studies have described many treatments, but none are evidence-based. However, the following treatment guidelines should be considered.
Emergency Treatment
1.Call an opthalmologist
2.Check direct and indirect light reflexes.
3.Timolol 0.5% 1–2 drops per eye
4.Consider retrobulbar hyaluronidase injection.
5.Ocular massage: press eyeball 10–15 seconds and suddenly release the pressure. Repeat for 3–5 minutes.
6.Treatment after transfer: IV acetazolamide 500 mg IV mannitol
IV heparin
Hyperbaric oxygen therapy IV corticosteroids
The purpose of ocular massage is to recanalize vessel by pressure differences. Timolol reduces ocular pressure, while acetazolamide decreases ocular pressures and increases retinal perfusion.
6.3.2 Retrobulbar Hyaluronidase Injection
The retrobulbar injection of hyaluronidase is the first treatment that should be considered in cases of blindness symptoms. Although its effectiveness remains to be confirmed, one study reported on its use to cure blindness. Not all doctors are familiar with this technique, but if this method can cure the complications, it should be performed. It is advisable that clinicians prepare for this tragic complication because the injection should be performed as soon as possible.
Technique: The distance between the anterior orbital margin and the retrobulbar space is at least 25 mm, so a long (38 mm) needle should be used. The needle length is generally 18G. The use of a 25G long needle or long cannula is also accept- able. The entry point should be the lateral part of the orbital rim and the orbital bone scratched by the needle tip to approach the retro- bulbar space. This is not a difficult procedure. We strongly suggest that clinicians practice the retrobulbar injection technique in a cadaver dissection workshop.
The proper dosage of the retrobulbar hyaluronidase injection has yet to be determined, but we recommend the injection of 1500 IU first, followed by 1500 IU. Previous studies described the injection of 400–800 USP, but since the United States markets 200 USP per bottle, the amount would be not enough. Since this is a tragic complication, authors like to recommend to inject as much as possible.
One author recently induced iatrogenic blind- ness by filler injections in rabbits and found that the retrobulbar injection of 3000 IU of hyaluronidase reversed the condition.
6.3.3 Emergency Kit
It is very important to have an emergency kit available because doctors tend to panic when filler complications such as skin necrosis and ocular complications occur.
There are emergency drugs for blindness and skin necrosis. It is advisable not to use nitroglycerin paste because of the risk of vessel choking, so a doctor should prepare their own medical knowledge.
Emergency Kit
1. Hyaluronidase 1500 IU
2. Timolol 0.25% eye drops
3. Nitroglycerine paste
4. IV – Prostaglandin E1 2 mL stored at
<5 °C
Dexamethasone Heparin 5000 U
5. Po: Opalmon
Ciprobay 260 mg
Methylprednisolone 4 mg
Aspirin 100 mg
6.5 Prevention
Prevention in such a tragic complication is critical. To achieve proper prevention, proper tech- niques must be used. Here are some suggestions.
6.5.1 Anatomy
The most important prevention is knowledge of the anatomy. Specifically, knowledge of the locations of the supratrochlear, supraorbital, and dorsal nasal arteries is extremely important.
6.5.2 Aspiration
Aspiration before a filler injection remains controversial. It is quite important when a needle tip punctures a large-diameter vessel, as small diameter vessels tend to shrink during aspiration, whereas large-diameter vessels do not. Also, when a needle is filled with filler, it is difficult to aspirate blood. Many studies have described that aspiration could not be performed by a small-diameter needle. Another point is that the needle tip moves during the injection, so complete prevention is impossible. However, for large-diameter vessels such as the supratrochlear, supraorbital, and dorsal nasal arteries, aspiration should be checked before the filler injections.
6.5.3 Big Cannula/Needle
Using a large versus small needle is controversial, but we strongly advise using a relatively large diameter cannula or needle (>23G). A larger-diameter needle needs a relatively low injection force. Actually, high pressure is one of the more common reasons for skin necrosis or blindness, so it is very important to control the pressure. Also, a smaller-diameter needle can more easily enter a vessel than a larger diameter needle, creating an embolism. Finally, a large diameter needle can be used to perform aspiration prior to the filler injection, so the use of a 23G or larger cannula or needle is highly recommended.
6.5.4 Compression
The easiest preventive method is compression of the arterial pathway. When the injection is made into the dorsum of the nose, bilateral compression should be applied at the dorsal nasal arteries. When injecting into the glabella, one should compress the supratrochlear pathway; when injecting into the forehead, one should compress the supratrochlear notch and supraorbital notch area. These two vessels are anastomosed to each other, so blocking both pathways is important.
6.5.5 Direction
Injection direction should be parallel to the artery and from the proximal to the distal direction. When the direction of injection is perpendicular to the artery, the probability of puncture is increased. And arteries tend to be shallow when they run distally, so less chance of puncture at distal location.
Thus, this rule should be followed, but in some places like glabella injections, it should be performed in opposite direction and more care should be taken.
6.5.6 Epinephrine
Epinephrine causes vasoconstriction, so it might be useful to avoid vascular compromise. But also it could not differentiate from the first sign of filler induced vascular occlusion which is pale skin change. Using epinephrine with lidocaine has advantages such as less pain, less bleeding, and less swelling.
6.5.7 Filler Injection Technique
Single bolus injection and linear threading technique have their own advantages and disadvantages. Linear threading technique cannot avoid vessel when the first passage tears the vessel. Thus, the single bolus technique is preferred. While performing the single bolus technique, vessel trauma should always be assessed first, and then a large volume should be injected at one location. In this method, there should be a 5 seconds gap between the puncture and injection to check for bleeding. When there is no bleeding, it is quite safe to inject. Most importantly, the avascular plane should be injected.
6.5.8 Gentle Injection
To inject with minimal pressure is absolutely important. To reduce pressure, injection should be performed using a big diameter needle and small volume syringe. Using a small diameter needle can help to improve the precision of injection, but it has a risk of vascular compromise. Gentle and smooth injection is absolutely important.
6.5.9 History
Previous operation history should be checked because normal vasculature has been changed during operation. For example, forehead augmentation with implant or rhinoplasty with implant would distort normal vasculature because of the implant and the capsule sur- rounding the implants. Also, previous operative lesions are not flexible, and the filler injected with more pressure increases the chance of vascular compromise.
In particular, previous open rhinoplasty patients with damaged columellar arteries should be careful of filler injections.
6.5.10 Injection by Cannula or Needle
The choice of needle or cannula is always controversial but should be chosen by considering advantages and disadvantages. Big diameter needle (23G) is preferred, but a lot of doctors like to use small diameter cannula. Advantages and dis- advantages are as follows:
Big diameter needles facilitate puncture of the precise layer. When the injector knows anatomical layers, it is definitely easy to use a big diameter to locate precise layer. But the needle tends to damage vessels, and when the tip is moved like cannula, more vessel trauma and more bruising can occur. Blunt microcannula has less chance of vessel trauma, but since the tip is blunt, it is difficult to locate the tip at a definite anatomical layer. One of the reasons of ocular complications of rhinoplasty is the use of a blunt cannula at infralobular entry point. Long and flexible blunt microcannula tip could locate subcutaneous layer instead of supraperiosteal layer. And this might cause embolism in the dorsal nasal artery and cause blindness
Therefore, 21G needle is preferred for nose augmentation. 21G is not flexible and has a big diameter and can be used to arrive at the exact layer. However, many doctors fear using this needle and use cannula and should be cautioned. Blunt microcannula can be flexible and locate subcutaneous layer, thus causing dorsal nasal artery trauma.
Preventive Guidelines (ABC Technique)
1.Anatomy: Always avoid subcutaneous layer.
2.Big needle: Bigger than 23G needle or cannula is recommended.
3.Compression: When inject territories of supratrochlear artery, supraorbital artery, dorsal nasal artery, Should com- press vessel pathway during injection
4.Direction: Needle should be inserted parallel to arterial pathway.
5.Epinephrine: Minimal use of epineph- rine is considerable.
6.Filler injection technique: Single bolus injection is a useful technique.
7.Gentle: Gentle injection with low pres- sure is extremely important.
8.History: Always check operation history.
Blindness is the most tragic complication and has no definite treatment until today. Therefore, preventive measures need to be elucidated to minimize this complication.
RESULT
In this thesis, I am focused on early and late complications of dermal fillers and their management. Most of the the complications are noted to be mild and transient. Serious adverse events are rare. Most adverse events can be avoided with proper planning and technique. Detailed understanding of facial anatomy, proper patient and product selection and appropriate technique can further reduce the risks. Adverse reactions always occur. The clinician must be prepared for these. A doctor should have tools available for effective treatment.
CONCLUSION
Minimally invasive techniques are in high demand. They provide a quick and reliable service for skin care rejuvenation. There is low risk of complications and high patient satisfaction. However, as with any surgical procedure, complications can occur and should be discussed before procedure initiation. Proper patient selection is the most important, as is significant knowledge of facial anatomy, skin pathology, and procedure techniques and options. Immediate recognition and treatment of complications can help minimise the likelihood of permanent consequences.
With significant patient demand and the rising rates of non trained practitioners performing these procedures, the recognition and management of complications will become increasingly important in clinical practice.
REFERENCES
1.Alcalay J et al. (2003) Late-onset granulomatous re- action to Artecoll. Dermatol Surg 29(8):859–862
2.Andre P (2004) Evaluation of the safety of a non-ani-mal stabilized hyaluronic acid (NASHA – Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol 18(4):422–425
3.Apte RS et al. (2003) Acute choroidal infarction fol- lowing subcutaneous injection of micronized dermal matrix in the forehead region. Retina 23(4):552–554
4.Bergeret-Galley C et al. (2001) The value of a new filler material in corrective and cosmetic surgery: DermaLive and DermaDeep.Aesthetic Plast Surg 25(4):249–255
5.Breiting V et al. (2004) A Study on Patients Treated with Polyacrylamide Hydrogel Injection for Facial Corrections. Aesthetic Plast Surg 28(1):45–53
6.Cheonis N (2002) New-Fill to treat facial wasting. Beta 15(2):10–15
7.Charriere G et al.(1987) Reactions to a bovine colla- gen implant. Clinical and immunologic study in 705 patients. J Am Acad Dermatol 21(6):1203–1208
8.Cooperman LS et al. (1985) Injectable collagen: a six-year clinical investigation. Aesthetic Plast Surg 9(2):145–151
9.De Cassia Novaes W, Berg A (2003) Experiences with a New Nonbiodegradable Hydrogel (Aquamid): A Pilot Study. Aesthetic Plast Surg 27(5):376–380
10.DeLustro F et al. (1987) Reaction to injectable colla- gen: results in animal models and clinical use. Plast Reconstr Surg 79(4):581–594
11.Ersek RA et al. (1997) Bioplastique at 6 years: clini- cal outcome studies. Plast Reconstr Surg 100(6): 1570–15741
12. Fagien S (2000) Facial soft-tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (autologen and dermalogen). Plast Reconstr Surg 105(1):362–373; discussion 374–375
13. Fernandez-Acenero MJ et al. (2003) Granulomatous foreign body reaction against hyaluronic acid: re- port of a case after lip augmentation. Dermatol Surg 29(12):1225–1226
14. Ficarra G et al. (2002) Silicone granuloma of the fa- cial tissues: a report of seven cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94(1):65–73
15. Fitzpatrick RE (1999) Treatment of inflamed hyper- trophic scars using intralesional 5-FU. Dermatol Surg 25(3):224–232
16. Friedman PM et al. (2002) Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tis- sue augmentation. Dermatol Surg 28(6):491–494
17. Hoffmann C et al. (1999) Adverse reactions after cosmetic lip augmentation with permanent biologi- cally inert implant materials. J Am Acad Dermatol 40(1):100–102
18. Homicz MR, Watson D (2004) Review of injectable materials for soft tissue augmentation. Facial Plast Surg 20(1):21–29
19. Lemperle G et al. (1998) PMMA-Microspheres (Artecoll) for long-lasting correction of wrinkles: re- finements and statistical results. Aesthetic Plast Surg 22(5):356–365
20. Lemperle G et al. (2003) Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg 29(6):573–587; discussion 587
21. Lombardi T et al. (2004) Orofacial granulomas af- ter injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med 33(2): 115–120
22. Lowe NJ (2003) Arterial embolization caused by in- jection of hyaluronic acid (Restylane). Br J Dermatol 148(2):379; author reply 379–380
23. Lowe NJ et al. (2001) Hyaluronic acid skin fillers: ad-verse reactions and skin testing. J Am Acad Dermatol 45(6):930–933
24. Lupton JR, Alster TS (2000) Cutaneous hypersensi- tivity reaction to injectable hyaluronic acid gel. Der- matol Surg 26(2):135–137
27. Micheels P (2001) Human anti-hyaluronic acid anti- bodies: is it possible? Dermatol Surg 27(2):185–91
28. Moody BR, Sengelmann RD (2000) Self-limited ad-verse reaction to human-derived collagen injectable product. Dermatol Surg 26(10):936–938
29. Moyle GJ et al. (2004) A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial li- poatrophy in persons with HIV infection. HIV Med 5(2):82–87
30. Protopapa C et al. (2003) Bio-Alcamid in drug-in- duced lipodystrophy. J Cosmet Laser Ther 5(3–4): 226–230
31. Rapaport MJ et al. (1996) Injectable silicone: cause of facial nodules, cellulitis, ulceration, and migration. Aesthetic Plast Surg 20(3):267–276
32. Raulin C et al. (2000) Exudative granulomatous reac- tion to hyaluronic acid (Hylaform). Contact Derma- titis 43(3):178–179
33. Reisberger EM et al. (2003) Foreign body granulomas caused by polymethylmethacrylate microspheres: successful treatment with allopurinol. Arch Derma- tol 139(1):17–20
34. Requena C et al. (2001) Adverse reactions to inject- able aesthetic microimplants. Am J Dermatopathol 23(3):197–202
35. Rudolph CM et al. (1999) Foreign body granulomas due to injectable aesthetic microimplants. Am J Surg Pathol 23(1):113–117
36. Rzany B et al. (2004) Anwendung von Polymilch- säure (New-Fill®) in der Ästhetischen Medizin: Ergebnisse des ersten deutschen Konsensustreffens an der Charité. Ästhetische Dermatologie: 3
37. Shafir R et al. (2000) Long-term complications of fa- cial injections with Restylane (injectable hyaluronic acid). Plast Reconstr Surg 106(5):1215–1216
38. Sklar JA, White SM (2004) Radiance FN: a new soft tissue filler. Dermatol Surg 30(5):764–768; discussion 768
39. Strom BL (1994) What is Pharmacoepidemiology? In: Strom BL (ed) Pharmacoepidemiology, 2nd edn. Wiley, New York
40. Tzikas TL (2004) Evaluation of the Radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg 6(4):234–239
41. Valantin MA et al. (2003) Polylactic acid implants (New-Fill®) to correct facial lipoatrophy in HIV-in- fected patients: results of the open-label study VEGA. Aids 17(17):2471–2477
42. Wang YB et al. (2003) [Clinically analyzing the possi- ble side-effects after injecting hydrophilic polyacryl- amide gel as a soft-tissue filler]. Zhonghua Zheng Xing Wai Ke Za Zhi 19(5):328–330
43. Waris E (2003) Alloplastic injectable biomaterials for soft tissue augmentation: a report on two cases with complications associated with a new material (Der- maLive) and a review of the literature. Eur J Plast Surg 26:350–355
44. Wölber L et al. (2005) Risiken Injizierbarer Füllmate- rialien, Ergebnisse der Injectable Filler Safety – Study. J Dtsch Dermatol Ges 3(Suppl.1):140
45. Park KH, Kim YK, Woo SJ, et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132(6):714–23.
46.Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol. 2006;34(4):363–4.
47. Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyal- uronic acid gel. Ophthalmic Plast Reconstr Surg. 2011;27(6):e152–5.
48. Chen Y, Wang W, Li J, Yu Y, Li L, Lu N. Fundus artery occlusion caused by cosmetic facial injections. Chin Med J. 2014;127(8):1434–7.
49. Kim SN, Byun DS, Park JH, et al. Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci. 2014;21(4):678–80.
50. Carle MV, Roe R, Novack R, Boyer DS. Cosmetic facial fillers and severe vision loss. JAMA Ophthalmol. 2014;132(5):637–9.
51. Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon OK. Iatrogenic retinal artery occlusion caused by cosmetic facial filler injections. Am J Ophthalmol. 2012;154(4):653–662.e1.
52. Kwon SG, Hong JW, Roh TS, Kim YS, Rah DK, Kim SS. Ischemic oculomotor nerve palsy and skin necro- sis caused by vascular embolization after hyaluronic acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333–4.
53. Kim EG, Eom TK, Kang SJ. Severe visual loss and cerebral infarction after injection of hyaluronic acid gel. J Craniofac Surg. 2014;25(2):684–6.
54. He MS, Sheu MM, Huang ZL, Tsai CH, Tsai RK. Sudden bilateral vision loss and brain infarction following cosmetic hyaluronic acid injection. JAMA Ophthalmol. 2013;131(9):1234–5.
55. Zhu GZ, Sun ZS, Liao WX, et al. Efficacy of retrobulbar hyaluronidase injection for vision loss resulting from hyaluronic acid filler embolization. Aesthet Surg J. 2017;38(1):12–22.
56. Chesnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44(3):435–7.
57. Nonomura S, Oshitari T, Miura G, Chiba A, Yamamoto S. A case of ophthalmic artery occlusion following injection of hyaluronic acid into the glabellar area. Nippon Ganka Gakkai Zasshi. 2014;118(9): 783–7.
58. Hu XZ, Hu JY, Wu PS, Yu SB, Kikkawa DO, Lu W. Posterior ciliary artery occlusion caused by hyal- uronic acid injections into the forehead: a case report. Medicine (Baltimore). 2016;95(11):e3124.
59. Lee WS, Yoon WT, Choi YJ, Park SP. Multiple cerebral infarctions with neurological symptoms and oph- thalmic artery occlusion after filler injection. J Korean Ophthalmol Soc. 2015;56(2):285–90.
60. Bae IH, Kim MS, Choi H, Na CH, Shin BS. Ischemic oculomotor nerve palsy due to hyaluronic acid filler injection. J Cosmet Dermatol. 2018;17:1016.
61. Ramesh S, Fiaschetti D, Goldberg RA. Orbital and ocular ischemic syndrome with blindness after facial filler injection. Ophthalmic Plast Reconstr Surg. 2018;34:e108–10.
62. Schelke LW, Fick M, van Rijn LJ, Decates T, Velthuis PJ, Niessen F. Unilateral blindness following a non-surgical rhinoplasty with filler. Ned Tijdschr Geneeskd. 2017;161(0):D1246.
63. Lee JI, Kang SJ, Sun H. Skin necrosis with oculomotor nerve palsy due to a hyaluronic acid filler injec- tion. Arch Plast Surg. 2017;44(4):340–3.
64. Chen W, Wu L, Jian XL, et al. Retinal branch artery embolization following hyaluronic acid injection: a case report. Aesthet Surg J. 2016;36(7):NP219–24.
65. Lin YC, Chen WC, Liao WC, Hsia TC. Central retinal artery occlusion and brain infarctions after nasal filler injection. QJM. 2015;108(9):731–2.
66. Kim YJ, Choi KS. Bilateral blindness after filler injection. Plast Reconstr Surg. 2013;131(2):298e–9e.
67. Sung MS, Kim HG, Woo KI, Kim YD. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthalmic Plast Reconstr Surg. 2010;26(4):289–91.
68. Hsiao SF, Huang YH. Partial vision recovery after iatrogenic retinal artery occlusion. BMC Ophthalmol. 2014;14:120.
69. Chou CC, Chen HH, Tsai YY, Li YL, Lin HJ. Choroid vascular occlusion and ischemic optic neuropathy after facial calcium hydroxyapatite injection- a case report. BMC Surg. 2015;15:21.
70. Marumo Y, Hiraoka M, Hashimoto M, Ohguro
H. Visual impairment by multiple vascular embolization with hydroxyapatite particles. Orbit. 2018;37(3):165–70.
71. Sung WI, Tsai S, Chen LJ. Ocular complications following cosmetic filler injection. JAMA Ophthalmol. 2018;136(5):e180716.
72. Roberts SA, Arthurs BP. Severe visual loss and orbital infarction following periorbital aesthetic poly-(L)-lac- tic acid (PLLA) injection. Ophthalmic Plast Reconstr Surg. 2012;28(3):e68–70.
73. Chen YH, Tsai YJ, Chao AN, Huang YS, Kao LY. Visual field defect after facial rejuvenation with botulinum toxin type A and polyacrylamide hydrogel injection. Plast Reconstr Surg. 2010;126(5):249e–50e.
74. Silva MT, Curi AL. Blindness and total ophthalmoplegia after aesthetic polymethylmethacrylate injection: case report. Arq Neuropsiquiatr. 2004;62(3B):873–4.
75. Kubota T, Hirose H. Permanent loss of vision following cosmetic rhinoplastic surgery. Jpn J Ophthalmol. 2005;49(6):535–6.